Article Text
Abstract
Background The costimulatory receptor 4-1BB (CD137, TNFRSF9) plays an important role in sustaining effective T cell immune responses and is investigated as target for cancer therapy. Systemic 4-1BB directed therapies elicit toxicity or low efficacy, which significantly hampered advancement of 4-1BB-based immunotherapy. Therefore, targeted delivery of 4-1BB agonist to the tumor side is needed for eliciting antitumor efficacy while avoiding systemic toxicity.
Methods We analyzed the immunostimulatory properties of a fibroblast activation protein (FAP)-targeted 4-1BB agonist (FAP-4-1BBL) by assessing tumor-infiltrating lymphocytes’ (TIL) activity from patients with non-small cell lung cancer and epithelial ovarian cancer.
Results Combination treatment with FAP-4-1BBL and T cell receptor stimulation by either anti-CD3 or T cell bispecific antibodies significantly enhanced TIL activation and effector functions, including T cell proliferation, secretion of proinflammatory cytokines and cytotoxicity. Notably, costimulation with FAP-4-1BBL led to de novo secretion of interleukin (IL)−13. This was associated with cytokine-mediated tumor cell apoptosis, which was partially dependent on IL-13 alpha 1/2 receptors and STAT6 phosphorylation.
Conclusions Our study provides mechanistic insights into T cell stimulation induced by FAP-4-1BBL in primary human tumors and supports the investigation of FAP-4-1BBL compound in early clinical trials.
- tumors
- immunology
- oncology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Cancer immunotherapy has shown major success in multiple cancer types during the last years.1 Indeed, antagonistic antibodies, which block coinhibitory checkpoint receptors on T cells such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) or its ligand PD-L1, can induce durable remissions and are now considered as one of the pillars of cancer therapy.2–4 Yet, treatment failure and resistance are seen in the majority of patients and therefore next-generation immunotherapy treatment regimens are urgently needed. Particularly, only a minority of patients with advanced non-small cell lung cancer (NSCLC) and epithelial ovarian cancer (EOC) demonstrate clinical responses to anti-PD-(L)1-blocking antibodies.4 Tumor-infiltrating lymphocytes (TILs) express a wide array of additional costimulatory and coinhibitory receptors that may serve as potential targets for immunotherapeutic interventions for cancer treatment.5 One such costimulatory receptor is the tumor necrosis factor (TNF) superfamily member 4-1BB that is expressed following activation of T cells6 and Natural Killer (NK) cells.7 Ligation of 4-1BB by its natural ligand (4-1BBL) provided by antigen-presenting cells (APCs) or by agonistic antibodies has been reported to enhance proliferation, effector functions, memory formation and survival in CD8+ T cells both in vitro and in vivo.8–10 4-1BB is considered to be an attractive drug target as 4-1BB upregulation in T cells is associated with encounter of antigen in the tumor, and 4-1BB provides a costimulatory signal to T cells. To date, two agonistic antihuman 4-1BB monoclonal antibodies (mAb), fully human IgG4 urelumab/BMS-663513 (NCT02534506) and humanized IgG2 utomilumab/PF-05082566 (NCT01307267), have entered phase I/II clinical trials and both antibodies showed evidence of clinical efficacy.11 Clinical progress, however, was compromised due to dose-limiting side effects including hepatotoxicity and cytokine release syndrome for urelumab12 or lack of single agent efficacy for utomilumab.10 Hence, strategies that deliver 4-1BB agonists specifically to the tumor site are required to reduce systemic toxicities while allowing for administration of clinically efficacious doses.13 Indeed, tumor-targeted 4-1BB agonists directed against epidermal growth factor receptor (1D8N/CEga1,14) or Her2 PRS-34315 16 have shown encouraging preclinical results of antitumor activity without eliciting substantial toxicity.
In this study, fibroblast activation protein (FAP)-targeted 4-1BBL (FAP-4-1BBL) was used to elicit 4-1BB agonistic T cell activation in human TILs.17 FAP is a membrane-bound serine protease restrictively found on reactive tumor stromal fibroblast, and highly expressed on common human epithelial cancers.18 Treatment with FAP-4-1BBL was combined with T cell bispecific antibodies (TCB), which simultaneously engage CD3ε on T cells and tumor antigen (TA) on cancer cells. In tumor mouse models, treatment with TCBs and FAP-4-1BBL decreased tumor growth while enhancing accumulation and activation of intratumoral CD8+ T cells.17 Stimulation of EOC tumor suspensions with FAP-4-1BBL in the presence of agonistic antihuman CD3 (αCD3) mAb led to increased 4-1BB expression and proliferation of CD8 T cells as well as increased proinflammatory cytokine production.17
Here, we further examined the potential of the FAP-4-1BBL agonist to deliver costimulatory signal to T cells on T cell receptor (TCR) engagement in primary human tumor samples from patient with lung and ovarian cancer to demonstrate T-cell specific cytokine production. As a polyclonal T cell stimulation, we have extended our analysis beyond αCD3 mAb and used TCBs which simultaneously engage CD3ε on T cells and a TA on cancer cells, such as carcinoembryonic antigen (CEA-TCB) or folate receptor 1 (FolR1-TCB). We were able to demonstrate that FAP-4-1BBL treatment significantly enhances T cell effector function in human primary tumor samples and leads to de novo interleukin (IL)-13 production by TILs, which enhances tumor cell apoptosis. This study provides an insight into mechanisms of 4-1BB agonism relevant for further translational research and supports clinical application of FAP-4-1BBL as a frontier in 4-1BB-based immunotherapy.
Material and methods
Reagents
FAP-4-1BBL, untargeted DP47-4-1BBL control (DP47-4-1BBL, DP47 is a germline control binder), antihuman CD3 (huIgG1 clone V9, αCD3), FolR1-TCB and CEA-TCB were generated by Roche Innovation Centre Zurich. Antihuman CD3ε (OKT3) and LEAF purified antihuman IL-13 (αIL-13) mAb were purchased from Biolegend. The anti-IL13Rα1 (GM1E7) blocking antibody was purchased from Abcam. Recombinant interleukin (rIL) rIL-13 (# 200-13) and rIL-4 (# 200-04) were obtained from Peprotech.
Cell lines
3T3 fibroblasts (ATCC CRL-1658) stably transfected with human FAP (3T3-huFAP clone 19 and 31 as 3T3-huFAPhigh and 3T3-huFAPlow, respectively) were cultured in Dulbecco's Modified Eagle Medium (DMEM,Gibco #42 430-082), supplied with 10% (v/v) fetal calf serum (FBS) and 5 µg/mL puromycin (InvivoGen, #ant-pr). Skov3 (human ovary adenocarcinoma), A549 (human lung carcinoma) and H460 (human lung pleura effusion carcinoma) were purchased from ATCC and cultured in DMEM (Sigma, D6429) supplemented with sodium pyruvate (1 mM), MEM nonessential AA (1X), L-glutamine (2 mM), penicillin/streptomycin (100 ng/mL), 2-mercaptoethanol (50 nM), ciproxin (1 mg/mL) and 10% FBS. NIH: OVCAR3, OAW42 (both EOC) were purchased from CLS Cell lines Service GmbH, Germany, and OVCAR8 (high-grade serous ovarian cancer) were obtained from Dr. Julia Schüler, Charles River, Germany. Cell lines were grown in RPMI (Sigma, #R0883) supplemented with sodium pyruvate (1 mM), MEM nonessential AA (1X), L-glutamine (2 mM), penicillin/streptomycin (100 ng/mL) and 10% FBS. MKN45 expressing a nuclear red fluorescence protein (MKN45-NRP clone 27) were generated at the Roche Innovation Centre Zurich by lentiviral transduction (Bioscience Essen, #4476) of the parental cell line MKN-45 (DMSZ ACC 409) and cultured in RPMI 1640 (Gibco, #42 401-042) supplied with 10% FBS, 2 mM L-alanyl-L-glutamine dipeptide, 2.5 ug/mL puromycine (InvivoGen, #ant-pr). Cells were confirmed to be negative for mycoplasma by PCR as described after every freeze-thaw cycle and then passaged every 2–3 days for a maximum of 10 passages.19
Quantitative FAP expression analysis of 3T3-huFAP cells
To determine the FAP expression, a quantitative analysis of indirect immunofluorescence staining by flow cytometry (Qifikit, Dako Agilent Technologies) was used. As detection antibody, the antihuman FAP mouse mAb clone F11-24 (Calbiochem #OP188) was used. The optimal saturation dose of 35 µg/mL (250 nM) was evaluated by a binding assay: 0.2×106 3T3-huFAP clone 19 cells (3T3-huFAPhigh) were seeded to a round-bottom 96-well plate (Greiner bio-one #650185) and incubated for 30 min in PBS containing 1:5000 diluted fixable Viability Dye eF450 (eBioscience, #65-0863-18). Cells were washed once and incubated with different titrated concentration of antihuman FAP mouse mAb clone F11-24 diluted in fluorescence-activated cell sorter (FACS) buffer (PBS supplemented with 2 mM EDTA, 0.1% potassium azide, 2% FCS) for 30 min at 4°C. Cells were washed four times with 200 µL/well FACS buffer, resuspended in FACS buffer containing 14 µg/mL polyclonal antimouse Fcγ-specific goat IgG antibody (Jackson Immunoresearch #115-545-071) and incubated for 30 min at 4°C. Cells were washed twice, fixed with PBS containing 1% formaldehyde for 15 min and acquired using MACS Quant X (Miltenyi Biotech) and Cytomat automation (ThermoFisher). For the Qifikit, 0.2×106 fibroblast or tumor cells were seeded to a round-bottom 96-well plate. Cells were stained for 30 min at 4°C with 1:5000 in PBS diluted Fixable Viability Dye eF660 (eBioscience #65-0864-18) and washed once with PBS. Cells were resuspended in FACS buffer containing 10 µg/mL antihuman FAP mouse mAb clone F11-24 and incubated for 1 hour at 4°C. Cells were washed three times. Cells and Qifikit beads were incubated in FACS buffer containing 1:50 diluted fluorescein isothiocyanate (FITC)-conjugated antimouse IgG second detection antibody (supplied with the Qifikit) for 30 min at 4°C. Cells and beads were washed twice, fixed with PBS containing 1% formaldehyde for 15 min and acquired using CantoII and DIVA software (BD Bioscience). Data were analyzed using FlowJo V.10.1.6 (Tree Star) and GraphPad Prism V.7.0a (GraphPad Software).
Isolation of healthy human blood cells and TILs
Human peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Histopaque-1077 (#Sigma, 10771) from buffy coats obtained from healthy blood donors (Blood Bank, University Hospital Basel, Switzerland). Fresh tumor tissues were collected from 18 patients with NSCLC and EOC undergoing surgery between July 2013 and September 2016 at the University Hospital Basel, Switzerland. Detailed patient characteristics are provided in online supplementary table S1. The analyzed samples comprised 17 solid tumors and 1 pleural effusion-derived single-cell suspensions from patients with NSCLC and EOC. The mean age of the patients was 68.4±10.7 years. All patients consented in writing to the analysis of their tumor samples. Staging was based on the seventh edition of the American Joint Committee on Cancer and Union for International Cancer Control (AJCC/UICC) tumor-node-metastasis staging system. Tumor lesions were mechanically dissociated and digested using accutase (PAA, PAA Laboratories, #L11-007), collagenase IV (Worthington, #LS004188), hyaluronidase (Sigma-Aldrich, #H6254) and DNAse type IV (Sigma-Aldrich, #D5025), directly after excision. Single-cell suspensions were prepared. All samples were stored in liquid nitrogen until further use.
Supplemental material
Antibodies and flow cytometry
The following human antibodies were used: anti-CD4-BV711 (clone SK3), anti-interferon γ (IFN-γ)-BV421 (4S.B3), anti-IL-13-APC (JES10-5A2), anti-PD1-PE-Cy7 (EH12.1), anti-BTLA-PE (J168-540), antiactive caspase 3-PE (C92-605) were purchased from BD; anti-CD45-PerCP Cy 5.5 (2D1), anti-CD3-APC eF780 (SK7), anti-4-1BB-FITC (4B4-1), anti-CD25-PE (CD25-4E3), anti-IL-2-PE (MQ1-17H12), anti-TNF-α-APC (MAb11), anti-Perforin-FITC (dG9), anti-LAG-3-FITC (3DS223H) and anti-TIGIT-PE (MBSA43) were purchased from eBioscience; antihuman CD3-PerCP Cy 5.5 (OKT3), antihuman CD4-BV421 (RPA-T4), antihuman CD8-BV510 (SK1), anti-CD8-BV605 (SK1), anti-4-1BB-APC (4B4-1), anti-pSTAT6 (Tyr641)-AF647 (A15137E), anti-IL13Rα1-APC (SS12B), anti-IL13Rα2-PE (SHM38), anti-TIM-3-BV421 (F38-2E2), anti-CD160-eF660 (BY55), antihuman CD25-PE Cy7 (BC96), anti-CD25-BV421 (BC96) and anti-Foxp3-AF647 (259D) were purchased from Biolegend; anti-EPCAM-FITC (HEA-125) was purchased from Miltenyi; anti-FOLR-1-APC (aa25-233) was purchased from Life-Span-Biosciences.
Cryopreserved tumor digests or PBMCs were thawed, washed as well as suspended in PBS, and blocked with Fc receptor-blocking agent (eBioscience). Dead cells were stained with Zombie UV Fixable Viability Kit (Biolegend, #423108) or Live/Death fixable Green dead cell stain (Molecular Probes, L34969). For surface staining, cells were washed, resuspended in FACS buffer (PBS supplemented with 2 mM EDTA, 0.1% sodium azide, 2% FCS), and stained with appropriate antibodies for 30 min (min) at 4°C. For intracellular (cytoplasmic) staining, cells were fixed and permeabilized using IC Fixation Buffer (eBioscience, #00-8222-49) and 1× permeabilization buffer (eBioscience, #00-8333-56). For staining of the nuclear proteins Ki-67 and Foxp3 a Foxp3/Transcription Factor Staining Buffer Set (eBioscience, #00-5523-00) was used for fixation/permeabilization, and cells were stained intracellularly for 30 min at room temperature (RT). After staining, cells were washed, resuspended in FACS buffer, and analyzed on a BD LSR Fortessa Cell analyzer (BD Bioscience) and BD FACS Diva Software version 7. In other experiments, data were acquired using MACS Quant X (Miltenyi Biotech) coupled to a Cytomat automation (ThermoFisher) flow cytometers. Collected data were further analyzed with FlowJo V.10.1.6 (Tree Star) and GraphPad Prism V.7.0a (GraphPad Software). All samples were analyzed by gating on viable cells followed by exclusion of duplets. All results show fluorescence on a biexponential scale.
Tumor sample characterization
All tumor samples were comprehensively characterized by multicolor flow cytometry. Dead cells and doublets were discriminated, and TILs were identified by gating for CD45+ (lymphocytes), CD3+ (T cells), CD4+ or CD8+ T cells and then divided into subpopulations based on the expression of PD-1, Tim-3, LAG-3, BTLA, CD160, TIGIT, CD25, Foxp3 (CD4+ T cells only) and 4-1BB. Tumor cells (CD45−EpCAMC+) were characterized for the expression of FolR1 by comparing the binding of a FolR1 specific antibody with its matched isotype control. Only samples that were positive for FolR1 expression were used for characterization of T cell activation and cytokine secretion on stimulation with FolR1-TCB and combination treatments.
IHC staining
All resection specimens were fixed in 10% neutral buffered formalin and immunohistochemistry (IHC) was performed on 4-μM-thick formalin-fixed and paraffin-embedded sections. H&E staining was performed using standard protocol. FolR1 IHC was performed with antihuman FolR1 antibody kit (Biocare Medical Germany, #BRI 4006K AA) by manual staining, following the manufacturer’s instructions. CD3 IHC was performed with antihuman CD3 antibody (RMAB005, Diagnostic Biosystem) at a 1:100 dilution in the LEICA BondMax platforms. Cytokeratin IHC was performed with antihuman cytokeratin antibody (Z06225, DAKO) at a 1:300 dilution in the LEICA BondMax platforms. FAP IHC was performed with antihuman FAP antibody (SP325, Spring Bioscience) at a 1:25 dilution in the Ventana Discovery XT platform.
Ex vivo treatment of PBMCs and tumor samples with αCD3, FAP-4-1BBL and FolR1-TCB
PBMCs, tumor digests or malignant effusions were thawed, washed and plated in 96-well flat bottom plates (BD Falcon, tissue culture treated, # 353072) in the presence of irradiated (45 Gy) 3T3-huFAPhigh cells at a ratio of 5:1 (100,000 CD3+ T cells to 20,000 3T3-huFAPhigh cells) in complete medium (PBMCs: RPMI 1640 supplemented with MEM nonessential AA (1×), L-glutamine (2 mM), penicillin/streptomycin (100 ng/mL), 10% FBS; tumor digests: DMEM supplemented with sodium pyruvate (1 mM), MEM non-essential AA (1×), L-glutamine (2 mM), penicillin/streptomycin (100 ng/mL), 2-mercaptoethanol (50 nM), ciproxin (1 mg/mL), 10% FBS). 3T3-huFAPhigh cells had a steadily FAP expression of ≥80 %, and expression was routinely determined by flow cytometry. The samples were cultured in the presence or absence of 10 nM anti-CD3 (αCD3), 10 nM anti-CD3 in combination with 1 nM FAP-4-1BBL (αCD3 +FAP-4-1BBL) or 10 nM anti-CD3 in combination with 1 nM DP47-4-1BBL control (αCD3 +DP47-4-1 BBL) for up to 120 hours. Where indicated, 2 pM of FolR1-TCB was used instead of anti-CD3 treatment. Activation of CD8+ and CD4+ positive T cells (CD45+CD3+) was determined by multicolor flow cytometry.
IncuCyte-evaluated tumor killing assay
To determine the role of FAP expression on tumor cell killing 5000 PBMCs were incubated in 96-well flat bottom culture plates (TTP, #92696) together with 5000 MKN45-NRP clone 27 and 10,000 50 Gy irradiated 3T3-huFAPlow or 3T3-huFAPhigh or 3T3 parental fibroblasts and in the presence of different concentrations of FAP-4-1BBL or CEA-TCB and IncuCyte Caspase-3/7 Green Apoptosis Assay Reagent (Bioscience Essen, #4440). Assay medium was RPMI 1640 supplemented with MEM non-essential AA (1×), 2 mM L-alanyl-L-glutamine dipeptide, 1 mM sodium pyruvate, 50 µM β-mercaptoethanol and 10% FBS. The growth of tumor cells (count of cell nuclei with red fluorescence/image) and cell apoptosis (count of cell nuclei with green fluorescence/image) were imaged over time (114 hours, images were collected every 3 hour) using an IncuCyte Zoom or S3 (Bioscience Essen). Further at endpoint, supernatants (SNs) were harvested and cytokines were analyzed by cytometric bead assay (CBA). Cells were also harvested at endpoint and T cell numbers and activation status were analyzed by flow cytometry.
CBA for cytokine determination
10 µL SN or standard was mixed with 10 µL capture bead diluent supplied with 0.2 µL of each capture bead (human Flex set from BD Bioscience: IFN-γ (E7), IL-2 (A4), granzyme B (D7), IL-6 (A7), IL-8 (A9), TNF (C4), IL-13 (E6)) in a 384-well flow cytometry plate (Corning #3830). After 1 hour incubation at RT and applied to an Eppendorf MixMate under constant rotations with 800 rounds/min in the dark, 10 µL PE detection reagent were added. After 2 hour incubation at RT and 800 rounds/min in the dark beads were washed four times with 80 µL wash buffer and beads were acquired using iQue Screener Plus (Satorius). Collected data were further analyzed with FlowJo V.10.1.6 (Tree Star), Microsoft office Excel and GraphPad Prism V.7.0a (GraphPad Software).
Caspase 3 apoptosis assay
To determine the killing capacity of FAP-4-1BBL-stimulated T cells derived from TILs, tumor digests were pretreated with medium only, 10 nM anti-CD3 (αCD3), or 10 nM anti-CD3 in combination with 1 nM FAP-4-1BBL (αCD3+FAP-4-1BBL) in the presence of 3T3-huFAPhigh cells 72 hours. T cells were then isolated and cultured with 30,000 CFSE-labeled Skov3 cells in the presence of 2 pM FolR1-TCB at an E:T ratio of 3:1 for 24 hours. Cell death of Skov3 cells was determined by flow cytometry by measuring activated caspase 3. For detection of IL-13-mediated apoptosis of tumor cells, 50,000 A549, Skov3, OVCAR-3, OVCAR-8 and OWA-42 were seeded in 96-well flat bottom culture plates and incubated with T cell culture SNs for 72 hours (±4 µg/mL αIL-13 or 5 µg/mL αIL-13Rα1 blocking antibodies) followed by flow cytometry analysis to detect cell death by measuring active caspase 3. The SN contained cytokines that were induced on treatment with medium only, anti-CD3 (αCD3), or anti-CD3 in combination with FAP-4-1BBL (αCD3+FAP-4-1BBL) in the presence of 3T3-huFAPhigh cells 72 hours. All assays were performed in duplicates.
ELISA and ProcartaPlex immunoassays
SN from cultured PBMCs and tumor digests were collected and analyzed for indicated cytokines by ELISA (BD Bioscience) or by ProcartaPlex Simplex/Multiplex Immunoassays (eBioscience), respectively, according to the manufacturer's protocol. Briefly, ELISA plates were coated with 100 µL capture antibody overnight at 4°C. The next day, plates were washed, blocked with assay diluent for 1 hour at RT followed by a second washing step. Samples were incubated for 2 hours. Working detector (detection Ab and SAv-HRP) was added, incubated for 1 hour, extensively washed again, and mixed with substrate solution. After addition of stop solution, plates were analyzed at 450 nm with λ correction 570 nm for accumulation of IFN-γ, IL-2, and TNF-α. Cytokine secretion was normalized to the amount of 100,000 CD3+ T cells present in cultures for all samples. For ProcartaPlex 6-plex immunoassay to detect IFN-γ, IL-2, TNF-α, IL-6, IL-10 and IL-13 and ProcartaPlex Human LAP (TGF beta 1), simplex immunoassay antibody-coated capture beads were washed and incubated with cell culture SN from TILs for 2 hours at RT followed by additional washing steps. Detection antibody was added and incubated for 30 min. Next, beads were washed and incubated under constant rotation with streptavidin-PE for 30 min at RT. Following final washing steps, beads were resuspended in reading buffer, sealed and incubated for 5 min with constant rotation at RT. Data were acquired using a Luminex200 system.
Isolation of primary tumor cells
To isolate primary tumor cells from patient samples, single cell suspensions of tumor digests were thawed and stained with antibodies against CD45 and Epithelial Cell Adhesion Molecule (EPCAM) for 20 min at 4°C. Following two washing steps, cells were resuspended in FACS buffer, filtered and CD45−EPCAM+ cells were sorted (BD FACS Aria III) into tubes containing assay medium. 4′,6-diamidino-2-phenylindole (DAPI) was used to detected dead cells.
STAT6 phosphorylation staining
For the detection of phosphorylated STAT6 on exposure to IL-13, tumor cell lines as well as sorted CD45−EPCAM+ primary tumor cells from patients with NSCLC and EOC were used. For stimulation, 100,000 cells were plated out in 96-well round-bottom plates (Corning, #353077) and cells were stimulated in a volume of 200 µL with either 30 ng/mL recombinant IL-13 (±4 µg/mL αIL-13; 5 µg/mL αIL-13Rα1-blocking antibodies) or 50 ng/mL IL-4 (±4 µg/mL αIL-13; 5 µg/mL αIL-13Rα1-blocking antibodies) and incubated for 15 min at 37°C. Stimulation was stopped by transferring the cells to 5 mL tube containing 2% PFA for 10 min at RT protected from light. Subsequently, cells were washed twice and permeabilized by adding dropwise while vortexing 400 µL precold (−20°C) True-Phos Perm buffer (Biolegend) in 5 mL polystyrene round-bottom tubes (Corning, #352052). Cells were kept at −20°C for 45 min. Next, cells were washed twice in PBS and transferred to a 96-well round-bottom plate for intracellular pSTAT6 (Tyr641) FACS staining.
Statistical analysis
The statistical analysis and graph preparation were performed using the software package Prism V.7.0 a (GraphPad Software, La Jolla, California, USA). Unless otherwise specified, experiments were performed without duplicates due to material restrictions. Functional data are representative of at least three experiments. Data were considered statistically significant with p<0.05 or lower.
Results
Costimulation with FAP-4-1BBL enhances activation of healthy donor-derived T cells
We first asked whether FAP-4-1BBL costimulation improves T cell activation and function in a FAP-dependent manner. We therefore generated 3T3 fibroblast cell lines with high or low surface expression of human FAP (3T3-huFAPhigh and 3T3-huFAPlow; figure 1A). The FAP protein density on the cell surface was determined via quantitative analysis of indirect immunofluorescence staining by flow cytometry (Qifikit) and a saturated concentration of antihuman FAP mouse IgG detection antibody (online supplementary figure 1A). The parental 3T3 cell line had no FAP expression (figure 1A). PBMCs from healthy donors were cultured with parental 3T3 fibroblasts spiked with different numbers of 3T3-huFAPhigh cells in the presence of αCD3 mAb only or αCD3+FAP-4-1BBL. Amount of IFN-γ produced by CD8+ (figure 1B) or by CD4+ T cells (online supplementary figure 1B) was measured by flow cytometry after intracellular cytokine staining (ICS) and was found to be proportional to the number of 3T3-huFAPhigh cells in the culture. In the absence of FAP+ 3T3 cells, FAP-4-1BBL did not stimulate IFN-γ production. This demonstrates that FAP-expressing cells are required to activate T cells using FAP-4-1BBL.
Supplemental material
Costimulation with fibroblast activation protein (FAP)-targeted 4-1BBL (FAP-4-1BBL) enhances T cell activation, proliferation and cytokine production. (A) Expression of FAP on 3T3 parental fibroblasts or clones stably transfected with human FAP was measured by quantitative analysis of indirect immunofluorescence staining by flow cytometry (Qifikit) by determining the number of specific antibody binding capacity (SABC). Shown is the calculated SABC of each cell line measured in technical triplicates as mean (SD) and one representative histogram showing overlay of unstained (red) and secondary antibody only (blue) controls and FAP staining (green). Mean fluorescence intensities are indicated. Significance was calculated with unpaired one-way analysis of variance (ANOVA) with multiple comparison Tukey test (**p<0.002, ****p<0.0001, ns: not significant). (B) 72 hours culture of healthy donor-derived peripheral blood mononuclear cells (PBMCs) in the presence of varying proportion of 3T3 fibroblasts expressing high levels of human FAP (3T3-huFAPhigh) among parental 3T3 cells treated with αCD3 or αCD3+FAP-4-1BBL. IFN-γ accumulation in CD8+ T cells by flow cytometry (intracellular staining) is shown. Statistical significance was analyzed by unpaired one-way ANOVA (****p<0.0001). (C–G) PBMCs of healthy donors (n=5) were cultured in medium only (unstim.) or treated with αCD3, αCD3+DP47-4-1 BBL (untargeted control), or αCD3+FAP-4-1BBL in the presence of 3T3-huFAPhigh cells for 72 hours. (C) Representative flow cytometry plots from one healthy donor under indicated treatment conditions show expression of activation markers (CD25, 4-1BB), proliferation (Ki67) as well as perforin and cytokine (interferon γ (IFN-γ), interleukin 2 (IL-2), tumor necrosis factor α (TNF-α)) accumulation in CD8+ T cells. (D–F) Summary of protein expression from five healthy donors. (G) Levels of cytokines in the supernatant of the cell cultures with indicated treatments measured by ELISAs. Statistical significance in graphs (D–G) was determined by paired one-way ANOVA (*p<0.05, **p<0.005). Horizontal bar represents mean.
To further investigate the effects of FAP-4-1BBL on T cell activation, proliferation and function, PBMCs from healthy donors were cocultured with 3T3-huFAPhigh cells in the presence of αCD3 mAb and FAP-4-1BBL. As a control, we used untargeted DP47-4-1BBL antibody that does not bind to FAP (online supplementary figure 1C). Expression of activation and proliferation markers, as well as cytokine and perforin accumulation in T cells was assessed by flow cytometry (representative FACS plots are shown in figure 1C). As expected, we observed a significant upregulation of activation markers CD25 and 4-1BB as well as proliferation marker Ki-67 in CD8+ and CD4+ T cells after stimulation with αCD3 mAb alone (figure 1D and online supplementary figure 1D). The combination treatment with αCD3+FAP-4-1BBL led to a further enhancement of CD25, 4-1BB and Ki67 expression. In the absence of FAP-targeting, as displayed by the exposure to αCD3+DP47-4-1 BBL, no difference was seen in comparison to αCD3 mAb alone (figure 1D and online supplementary figure 1D). The amount of CD25 and 4-1BB protein per cell, expressed as mean fluorescence intensity (MFI, online supplementary figure 1E), was also higher in the CD8+ and CD4+ T cells exposed to αCD3+FAP-4-1BBL when compared with αCD3 mAb alone or αCD3+DP47-4-1 BBL. Treatment with the αCD3+FAP-4-1BBL did not increase the expression of perforin in CD8+ or PD-1 in CD8+ and CD4+ T cells (figure 1E and online supplementary figure 1F). Additionally, 4-1BBL agonism in T cells amplified production of the proinflammatory cytokines IFN-γ, IL-2 and TNF-α by CD8+ and CD4+ T cells, compared with the treatment with αCD3 alone or with αCD3+DP47-4-1 BBL (figure 1F and online supplementary figure 1G). The amount of cytokine per cell (MFI) was also higher in the CD8+ and CD4+ cells exposed to αCD3+FAP-4-1BBL when compared with αCD3+DP47-4-1 BBL control (online supplementary figure 1H). The cytokine release was further confirmed by ELISA (figure 1G). Additionally, we did not detect an increase of proapoptotic receptor FAS-L and protein TRAIL-2 after FAP-4-1BBL stimulation (online supplementary figure 1I).
Together, we demonstrate that costimulation with FAP-4-1BBL increases activation, proliferation and cytokine production in CD8+ and CD4+ T cells derived from healthy donors’ PBMCs.
FAP-4-1BBL activity improves tumor cell killing
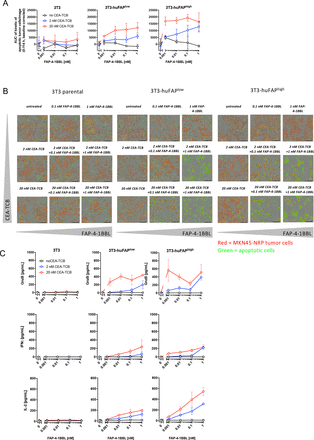
To determine whether costimulation with FAP-4-1BBL improved tumor cell killing, we tracked tumor cells undergoing apoptosis using live fluorescence microscopy imaging over time (IncuCyte assay, online supplementary figure 2A). MKN45 tumor cell line (FAPneg, online supplementary figure 2B) was transduced to express a NRP allowing a direct tumor cell count over time. MKN45-NRP tumor cells were cultured with 3T3-huFAPhigh, 3T3-huFAPlow and parental 3T3 fibroblasts in the presence of healthy donors-derived PBMCs. The latter were stimulated with TCB which engages CD3ε on T cells and CEA-related cell adhesion molecule 5 expressed on tumor cell (CEA-TCB, online supplementary figure 2A) in the presence of increasing FAP-4-1BBL concentrations. Tumor cell apoptosis was found to be proportional to the FAP expression levels (quantification in figure 2A and online supplementary figure 2C). Representative videos of the cell cultures are presented in figure 2B, with baseline of the assay showed in online supplementary figure 2D. Little effect on tumor cells was observed in the presence of FAP-negative parental 3T3 fibroblasts (figure 2A) even in the presence of high CEA-TCB concentration. Conversely, stimulation with high concentration of FAP-4-1BBL in the absence of CEA-TCB or FAP did not lead to increase in tumor cell apoptosis. These results show that FAP-4-1BBL can improve tumor cells killing also at low FAP expression if T cells receive sufficient TCR stimulation. Tumor cell apoptosis correlated with increased CD4+ and CD8+ T cell counts at end point as well as their activation status, measured by expression of CD25 and 4-1BB (online supplementary figure 2E). We also noted increased production of granzyme B, IFN-γ and IL-2 correlating with observed tumor cell killing (figure 2C). Together, we demonstrate that costimulation with FAP+ fibroblast crosslinked-FAP-4-1BBL in the presence of CEA-TCB compound improves T cell activation and cytokine production and leads to increased tumor cell apoptosis.
Supplemental material
Fibroblast activation protein (FAP)-targeted 4-1BBL (FAP-4-1BBL) activity improves tumor killing. (A) FAP-4-1BBL dose-dependent increase of induced tumor cell apoptosis (caspase 3/7 activation) measured by IncuCyte live fluorescence microscopy imaging after treatment with carcinoembryonic antigen T cell bispecific antibodies (CEA-TCB) and FAP-4-1BBL and different FAP-expressing 3T3 fibroblasts. Shown is the area under the curve (AUC) of killing kinetics measured every 3 hours between 0 and 112 hours versus the concentration of FAP-4-1BBL in nM. Each curve indicates a different CEA-TCB concentration and each blot a different 3T3 clone. Data were baseline corrected to untreated control. Shown is the mean (SD) of technical triplicates per condition. (B) Representative videos of imaging tumor cell count (red fluorescence) and cell apoptosis (caspase 3/7 detection reagent displayed as green fluorescence) after treatment with CEA-TCB and FAP-4-1BBL. Shown is one image out of 12 analyzed images per condition (four image per technical triplicates). Images were taken with a 10 × magnification. (C) At endpoint, supernatants were harvested from each technical triplicate and cytokines were measured via cytometric bead assay. Shown are mean (SD) of different cytokines (granzyme B, interferon γ (IFN-γ) and interleukin 2 (IL-2)) in pg/mL versus the concentration of added FAP-4-1BBL in nM. Each curve indicates a different CEA-TCB concentration and each blot a different 3T3 clone.
Costimulation with FAP-4-1BBL enhances T cell activation in primary tumor samples
To assess the activity of FAP-4-1BBL in T cells derived from human cancer, we used TILs freshly obtained from primary tumors of patients with NSCLC and EOC and one pleural effusion (online supplementary table 1). Tumor-resident T cells exhibit a highly dysfunctional state, hallmarked by the overexpression of inhibitory receptors.20–23 Consistently, CD8+ and CD4+ T cells in our experimental cohort expressed high levels of inhibitory receptors including PD-1, TIGIT, TIM-3, CD160, BTLA and Lag-3 (figure 3A and online supplementary figure 3A). Of note, TILs were found to express low but consistently detectable levels of 4-1BB protein (figure 3B). We further evaluated FAP expression in the NSCLC/EOC cohort by IHC using consecutive tissue sections from 11 tumor samples. FAP presence in tumor stroma was confirmed in all tumors included in the experimental cohort (figure 3C and online supplementary table 2), while expectedly the frequency of FAP-positive cells substantially varied among patients. To allow constant FAP expression level for the subsequent experiments, TILs from digested tumor samples were spiked with 3T3-huFAPhigh fibroblasts to ensure a final effector to FAP+ fibroblast cells ratio of 5:1. To investigate reinvigoration of the intratumoral dysfunctional T cells through 4-1BB, we exposed TILs from patients to FAP-4-1BBL in the presence of either αCD3 mAb or clinically relevant FolR1-TCB, expressed commonly by tumor cells.22 The expression of FolR1 in the cohort was confirmed by IHC staining (online supplementary figure 3B and online supplementary table 2).
Supplemental material
Supplemental material
Costimulation with fibroblast activation protein (FAP)-targeted 4-1BBL (FAP-4-1BBL) enhances T cell function in primary human tumors. (A) Expression of exhaustion markers programmed cell death protein 1 (PD-1), TIGIT and Tim-3 determined by flow cytometry on CD8+ and CD4+ tumor-infiltrating lymphocytes (TILs) from primary epithelial ovarian and lung tumors. (B) Expression of 4-1BB determined by flow cytometry on CD8+ and CD4+ TILs from primary epithelial ovarian and lung tumors. (C) Representative H&E staining (top left) and FAP expression (bottom left) determined by immunohistochemistry staining. Summary of FAP expression in the cohort of 11 patients with non-small cell lung cancer/epithelial ovarian cancer (right, bar represents mean). (D–I) Tumor cell suspensions were cultured in medium only (unstim.) or treated with αCD3 alone, αCD3+FAP-4-1BBL, FolR1 alone or FolR1 +FAP-4-1BBL in the presence of 3T3-huFAPhigh cells for 72 hours. (D) Presence of interferon γ (IFN-γ), interleukin (IL)-2 and tumor necrosis factor α (TNF-α) in the culture supernatant from indicated treatments was measured by ELISA. (E–F) Flow cytometry analysis of cytokine accumulation in (E) CD8+ and (F) CD4+ TILs. (G) Presence of suppressive cytokines IL-10, IL-6, transforming growth factor-β (TGF-β) was measured via multiplex immunoassays. Level of significance set at p<0.05, ns: not significant. (H) FAP-4-1BBL-mediated increase of Bcl-2 expression in CD8+ and CD4+ TILs. (I) Perforin accumulation measured by intracellular flow cytometry staining in CD8+ TILs under indicated treatments. Data in D–I were analyzed by paired one-way analysis of variance (*p<0.05, **p<0.005, ***p<0.0005) with Tukey post hoc test for multiple comparisons. (J) Tumor cell digest was preactivated for 72 hours with αCD3 alone or αCD3+FAP-4-1BBL in the presence of 3T3-huFAPhigh cells. Afterwards, T cells were harvested and cocultured with CFSE-labeled Skov3 tumor cells at E:T ratio of 3:1 in the presence of 2 pM FolR-1-TCB for 24 hours. Apoptosis in Skov3 cells was determined by flow cytometry by measuring active caspase 3. The graph shows the fold change of caspase 3 with αCD3 preactivated TILs versus αCD3+FAP-4-1BBL pretreated TILs. Data were analyzed by paired t-test (*p<0.05). MFI, mean fluorescence intensity.
In contrast to healthy donor PBMCs, we did not see upregulation of activation markers (CD25, 4-1BB) and proliferation marker Ki67 in CD8+ or CD4+ TILs (online supplementary figure 3C,D, respectively) treated with FAP-4-1BBL and αCD3 or FolR1-TCB. Nevertheless, we detected high levels of proinflammatory cytokines such as IFN-γ, IL-2 and TNF-α in the culture SNs of TILs treated with FAP-4-1BBL + αCD3 or FAP-4-1BBL+FolR1 TCB (figure 3D). We did not observe substantial cytokine release in αCD3-treated or FolR1-TCB-treated controls (figure 3D). ICS confirmed that both CD8+ and CD4+ TILs were producers of IFN-γ, IL-2 and TNF-α (figure 3E) after treatment with FAP-4-1BBL. In addition to enhanced proportion of T cells expressing the cytokines, MFI was also increased after costimulation with FAP-4-1BBL + αCD3 (online supplementary figure 3E,F). We further showed that neither the frequency of CD4+ CD25high Foxp3+ Treg cells (online supplementary figure 3G) nor levels of anti-inflammatory cytokines IL-10, IL-6 and TGF-β were affected by the costimulation with FAP-4-1BBL (figure 3G). Compatible with healthy donor PBMCs, costimulation with FAP-4-1BBL did not significantly modulate the expression level of PD-1 in intratumoral CD8+ and CD4+ T cells (online supplementary figure 3H). Furthermore, cross linking with FAP-4-1BBL enhanced the expression of Bcl-2, a protein critical for lymphocyte survival, in both CD8+ and CD4+ TILs (figure 3H). Additionally, there was no increase in proapoptotic receptor FAS-L and protein TRAIL-2 expression in CD4 and CD8 TILs treated with FAP-4-1BBL (online supplementary figure 3I). These results collectively show that FAP-4-1BBL treatment specifically enhances proinflammatory cytokine production and survival in TILs.
To further assess the cytolytic potential of FAP-4-1BBL-activated T cells, we measured perforin production in TILs and found an increase in perforin in CD8+ T cells after treatment with FAP-4-1BBL + αCD3 or FAP-4-1BBL+FolR1 TCB compared with the treatment with αCD3 or FolR1-TCB alone (figure 3I and online supplementary figure 3J). To directly demonstrate tumor killing, TILs were pretreated with αCD3 or FAP-4-1BBL+αCD3 in the presence of 3T3-huFAPhigh cells for 72 hours. T cells were then harvested and cocultured with CFSE-labeled Skov3 tumor cells for 24 hours at a 3:1 E:T ratio in the presence of 2 pM FolRI-TCB. We noted increased levels of activated caspase 3 in Skov3 cells in cultures with TILs prestimulated with αCD3+FAP-4-1BBL compared with αCD3 only (figure 3J), suggesting an enhancement of cytolytic cell function mediated by FAP-4-1BBL costimulation.
Taken together, we demonstrate that dysfunctional CD8+ and CD4+ TIL populations from patients with NSCLC and EOC can undergo functional reinvigoration with FAP-4-1BBL costimulation, which is characterized by enhanced survival, cytokine production and tumor killing capacity.
FAP-4-1BBL induces IL-13 secretion in CD4+ and CD8+ TILs
Analysis of the secretome specifically released on FAP-4-1BBL costimulation revealed a significant increase in secretion of IL-13 cytokine in the culture SN in comparison to αCD3 or FolR1-TCB treatments alone (figure 4A). Increased IL-13 production after 4-1BB agonism was also previously observed in the IncuCyte cultures with healthy donors’ PBMCs (online supplementary figure 4A). Furthermore, ICS revealed that CD8+ and CD4+ TILs were producers of IL-13, which was significantly increased on costimulation with FAP-4-1BBL compared with αCD3 or FolR1-TCB stimulation alone (figure 4B). Additionally, CD8+ TILs produced higher levels of IL-13 compared with CD4+ TILs on FAP-4-1BBL costimulation. Notably, while IFN-γ, IL-2 and TNF-α secretion was significantly enhanced after αCD3 or FolR1-TCB treatments alone (when compared with unstimulated cells, figure 3D,E), increased IL-13 secretion was noted only on 4-1BB engagement on T cells (figure 4B). This implies that targeted 4-1BB agonism leads to an amplification of the inflammatory response typically induced by αCD3 engagement and, importantly, drives a T cell differentiation program which is associated uniquely with IL-13 secretion.
Supplemental material
Fibroblast activation protein (FAP)-targeted 4-1BBL (FAP-4-1BBL) induces secretion of interleukin (IL)-13 by CD4+ and CD8+ T cells which induces tumor cell apoptosis. (A) Presence of IL-13 in the supernatant (SN) in the cultures of primary tumor cells treated with FAP-4-1BBL in combination with αCD3 (left) or FolR-1-TCB (right). Data were analyzed by paired t-test (*p<0.05). (B) IL-13 expression by CD8+ TILs and CD4+ TILs determined by intracellular flow cytometry staining. Data were analyzed by paired one-way analysis of variance (ANOVA; **p<0.005, ***p<0.0005). (C) Surface expression or IL-13Rα1/α2 in lung and ovarian cancer cell lines. (D) IL-13Rα1/α2 surface expression of primary tumor cells of patients with non-small cell lung cancer and epithelial ovarian cancer. (E) Levels of STAT6 phosphorylation (at Tyr641, indicative of IL-4/IL-13 signaling) measured by flow cytometry in primary tumor cells treated with rIL-4, rIL-13 or rIL-13 and IL-13Rα1 blocking antibody. (F) Tumor cell digest was treated for 72 hours with medium only (unstim.), αCD3 alone or αCD3+FAP-4-1BBL in the presence of 3T3-huFAPhigh cells. Afterwards, SNs from the cultures were transferred to the ovarian and lung tumor cell lines and αIL-13 or αIL-13Rα1 blocking antibodies were added for 72 hours. Apoptosis in tumor cells was determined by flow cytometry by measuring active caspase 3. Shown are also representative plots and gating of active caspase 3+ cells of cell line A549. Data in E and F were investigated by paired two-way ANOVAs with Tukey post hoc test for multiple comparisons (*p<0.05, **p<0.005, ***p<0.0005, ****p<0.0001). MFI, mean fluorescence intensity.
IL-13 secreted by TILs mediates tumor cell apoptosis
We further assessed the impact of IL-13 secretion on the antitumor response. To this end, we investigated the expression of IL-13R in tumor cell lines and patient-derived tumor cells. Both tumor cell lines (lung: A549; ovarian: OVCAR-3, OVCAR-8, OWA42 and Skov3) as well as freshly obtained patient-derived tumor cells expressed substantial levels of the IL-13Rα1 (figure 4C). While IL-13Rα2 was not expressed in tumor cell lines, primary tumor cells showed weak and variable expression of IL-13Rα2 (figure 4D). To determine whether expressed IL-13Rα1 was functional, we assessed phosphorylation of STAT624 25 by flow cytometry. Phosphorylated STAT6 was detected on exposure to IL-13 in all tumor cell lines (online supplementary figure 4B) as well as patient tumor cells (figure 4E). IL-4, which is known to induce IL-13Rα1 signaling, was used as a positive control.24 Importantly, STAT6 phosphorylation was significantly reduced on treatment with αIL-13Rα1 blocking antibodies in all tested tumor cell lines and primary tumor cells (online supplementary figure 4B and figure 4E, respectively). This confirms that the expressed IL-13R is functionally involved in transmitting IL-13 signals in tumor cells. Tumor cell lines were then exposed to SNs from PBMCs treated with αCD3+FAP-4-1BBL. Expression of active caspase 3 was significantly increased in tumor cells incubated with the SN from PBMC cultures treated with αCD3+FAP-4-1BBL compared with αCD3 alone treated SNs (figure 4F). Importantly, the expression of active caspase 3 was reduced in the presence of αIL-13 or αIL-13Rα1 blocking antibodies in four out of five tested cell lines (figure 4F). These results demonstrate an essential role of IL-13 in mediating tumor cell killing.
In conclusion, we demonstrate that 4-1BB costimulated T cell acquire the unique function of IL-13 secretion, which further mediates tumor cell apoptosis.
Discussion
As a member of TNF superfamily, 4-1BB receptor requires higher order clustering to deliver cell costimulation.26 Both of the agonistic 4-1BB mAb used previously in clinical trials26 (urelumab, IgG4 and utomilumab, IgG2a) rely on eliciting such higher order clustering by FcγRIIb-mediated crosslinking. Unfortunately, with this approach, 4-1BB activity was systemic and accompanied by high liver toxicity, while administration of lower but safe dose of the mAb was not enough to observe clinical benefits.10 To circumvent issues of FcγR binding and to reduce systemic toxicity, we utilized a 4-1BB agonistic antibody fusion protein with the following properties: (1) human IgG1 with abrogated FcRγ binding capacity mediated by P329G L234A L235A mutations in the Fc fragment,27 (2) a trimeric split 4-1BBL that binds 4-1BB, and (3) a monovalent Fab fragment that binds specifically FAP with high affinity.17 Such design enables improved in vivo T cell stimulation through the hypercrosslinking of 4-1BB expressed by T cells and FAP expressed by tumor stroma while avoiding FcγR-mediated toxicities. In our cohort of tumors from patients with NSCLC and EOC, FAP expression was detected in all samples and in 10/11 patients there was between 15% and 36% of FAP expression on stromal cells. Presence of FAP is required for activity of FAP-4-1BBL, since no effects of costimulation in healthy donor-derived PBMCs in the absence of FAP-expressing cells are observed. This result is in agreement with previous observations that the FAP-4-1BBL construct specificity colocalizes with FAP-expressing tissues in a rhesus monkey bearing natural colorectal carcinoma.17
Providing costimulation (‘signal 2’) to antitumor T cells has long been recognized as a promising approach for cancer immunotherapy.8 28 4-1BB costimulation only increases T cell activation if combined agonistic CD3 stimulus by either αCD3 mAb or a TCB (signal 1).14 26 This maneuver results in polyclonal T cell activation, release of cytotoxic granules as well as cytokines and leads ultimately to enhanced tumor killing.29 We applied CEA-TCB30 31 which is in clinical trials currently (NCT03866239) and FolR1-TCB22 for this purpose. CEA and FolR1 antigens are expressed at low levels in healthy tissues and become overexpressed in many cancers, including NSCLC, breast, colorectal, gastric and pancreatic tumors.32–34 CEA-TCB treatment has shown efficacy in mouse tumor models31 and early clinical trials.35 Therefore, our study provides validation of combining 4-1BB-targeted therapy with TCR-directed compounds by showing efficacy of such treatment in primary tumor suspensions.
The importance of 4-1BB signaling in antitumor responses has been also demonstrated in humanized mouse models, chimeric antigen receptors (CAR)-T cells and in primary TIL suspensions. In a humanized mouse tumor xenograft model, FAP-4-1BBL and CEA-TCB treatment resulted in increased intratumoral CD8+ T cell accumulation and tumor reduction.17 CAR-T cells,36 carrying the intracellular 4-1BB signaling domain, show superior systemic persistence37 and reduced CAR T cell exhaustion compared with CAR T cells carrying CD28-derived intracellular signaling domain.38 Therefore, studies exploring mechanism of 4-1BB engagement are desired to fully understand clinical potential of 4-1BB in order to design efficient immunotherapies.
In our study, TILs were shown to be in a dysfunctional state characterized by the expression of the immunoinhibitory receptors PD-1, TIGIT, Tim3, LAG3, CD160 and CTLA-4 on T cells.21–23 39 Such TILs have diminished effector function, characterized by impaired production of cytokines and effector granules.20 23 40 Importantly, 4-1BB was found to be coexpressed by CD8 TILs displaying high levels of PD-1 (PD-1T cells, found almost exclusively in tumors), a CD8 TIL subset shown to be tumor reactive.23 Hence, 4-1BB agonism can provide the costimulatory signal necessary for tumor-specific, exhausted T cells to induce specific antitumor responses.
4-1BB stimulation was shown previously to improve T cell proliferation,41 survival42 killing41 43 and proinflammatory cytokine secretion.41–44 In agreement with these findings, we noted that secretion of inflammatory cytokines such as IFN-γ and TNF-α, which also mediate tumor cell apoptosis, were found to be induced on αCD3 or FolR1-TCB stimulation and further amplified by costimulation with FAP-4-1BBL in primary human tumor suspensions. More importantly, we observed that T cells of TILs costimulated with FAP-4-1BBL uniquely secreted IL-13, which did not occur after stimulation with αCD3 or FolR1-TCB alone. To mechanistically dissect the contribution of 4-1BB costimulation, we focused on further investigating the role of IL-13, the cytokine uniquely induced in treatments containing 4-1BB agonism, and we sought to determine the consequences of IL-13 secretion on tumor cells. IL-13 is reported to mediate antiproliferative as well as antiapoptotic effects in cancer cells.45–48 We found that IL-13 enhances tumor cell apoptosis in primary cancer samples from patients with NSCLC or EOC. This is likely due to direct effect of IL-13 on tumor cells, given the cancer cell lines tested as well as primary tumor digests express functional IL-13Rα1, capable of transmitting IL-13-mediated signals.25 Interestingly, expression of IL-13Rα2, which can function as a decoy receptor,25 was not observed in cancer cell lines but was detected in some of the patients with primary tumors, which is in line with published studies.49 This might suggest a possible escape mechanism, in which tumor cells actively downregulate functional IL-13Rα1 and upregulate decoy IL-13Rα2 to avoid proapoptotic signals.
Interestingly, IL-13R may be expressed on myeloid cell populations found in the lung, such as alveolar macrophages and dendritic cells,50 as well as tumor-associated macrophages. Though IL-13 promotes alternative activation of macrophages (which can inhibit T cell expansion),51 we did not notice a decrease in proliferation of CD8 T cells derived from the tumor suspensions. Another regulatory population that could potentially be affected by 4-1BB activation are regulatory T cells (T regs). Though we are not able to formally rule out any coactivation of regulatory cells, we argue that 4-1BB ligation by FAP-4-1BBL results overall in a beneficial effect by mainly activating effector cells. First, 4-1BB expression on T regs is rather low compared with effector CD4 and CD8 T cells. Second, we do not observe an increase in anti-inflammatory cytokines such as IL-10 and TGF-β after FAP-4-1BBL treatment. Third, proliferation of CD8 T cells was maintained by FAP-4-1BBL in T cells derived from healthy donors and primary tumor suspensions.
Both CD4+ and CD8+ TILs secreted IL-13, yet the secretion of IL-13 was more pronounced in CD8+ TILs. This is in line with higher 4-1BB expression on CD8+ than CD4+ T cells in our cohort and published studies showing that 4-1BB stimulation has more pronounced effect on CD8+ T cells when compared with CD4+ T cells.52 Notably, in mice, treatment with an agonistic anti-4-1BB antibody enhanced IL-13 secretion by CD8+ T cells.53 In addition, IL-13 was shown to maintain a balanced Th1/Th2 immune response and prevent inflammation triggered by a hyperpolarized Th1 response during the treatment of Her2-positive advanced or metastatic solid tumors.12 Although IL-13 is considered as classical Th2 cytokine, often coexpressed with IL-4 and released by helper T cells, it has also been reported that IL-13 can be secreted by IFN-γ+ Th1 and IL-17+ Th17 cells as well as CD8+ T cells, independent of IL-4.27 28 Our study identifies IL-13 as a mediator of tumor cell apoptosis as a consequence of 4-1BB signaling in TILs.
In summary, our work provides extensive preclinical characterization of FAP-4-1BBL costimulation in human primary tumor tissues and suggests an important role of IL-13 in FAP-4-1BBL-induced tumor cell apoptosis. Additionally, our results provide a strong rationale for combining FAP-4-1BBL with TCBs in early clinical trials. Therefore, this study paves way for an off-the shelf tumor-targeted approach in 4-1BB-based therapy as a mean to advance treatment of solid tumors.
Acknowledgments
The authors thank the FACS Core Facility of the DBM of the University of Basel. They also thank all the patients who allowed the use of their material and made this work possible.
References
Footnotes
MT, FU and CC contributed equally.
Correction notice This article has been corrected since it was published Online First. Equally contributing status was added to authors Trüb M., Uhlenbrock F. and Claus C. Senior authorship status was added to authors Läubli H., Kashyap A.S and Zippelius A.
Contributors FU, CC, VK, MB, MA, CK, PU and AZ conceived the idea for the study. MTr, MTh, ASK, FU, HL, CC and AZ interpreted the data, made the figures and wrote the manuscript. FU, HL, MTh, CC, DT and AZ planned the experiments. FU, MTr, MTh, RA, CC and PH performed and analyzed the experiments. DL, VK, KDM, GC, MW, VH-S, RR and SSP provided samples. HL, SR and AZ collected the clinical data. CC, MA, CK and CF-K generated and provided key reagents. All authors reviewed and approved the manuscript.
Funding This work was supported by grants from the Swiss National Science Foundation (320030_188576), the Wilhelm Sander-Foundation, the Cancer League Basel, the Basel Translational Medicine Hub, the Roche Innovation Fund and the Research Funds of the University Basel.
Competing interests HL, SR and AZ received research funding from Bristol-Myers Squibb. FU, PH and AZ received research funding from Roche Innovation Center Zurich. PU, CK, MB, VK, MA, CC and CF-K are employed by Roche Innovation Center Zurich and declare ownership of stock and patents with Roche. RA was employed by Roche Innovation Center Zurich. CC declares ownerships of patents. AZ received honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Hoffmann–La Roche, NBE Therapeutics, Secarna, ACM Pharma and Hookipa. AZ maintains non-commercial research agreements with Hoffmann–La Roche. AZ maintains further non-commercial research agreements with NBE Therapeutics, Secarna, ACM Pharma, Hookipa, and BeyondSpring.
Patient consent for publication Not required.
Ethics approval The study was approved by the local ethical review board (Ethikkommission Nordwestschweiz, EK321/10), and all patients consented in writing to the analysis of their tumor samples.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Any questions regarding published results, reagents or method should be directed to: Alfred Zippelius, Laboratory of Cancer Immunology, University of Basel/University Hospital of Basel, Department of Biomedicine, Hebelstrasse 20, 4031 Basel, Switzerland, phone: +41 61 265 23 55, alfred.zippelius@usb.ch, or Christina Claus, Roche Innovation Center Zurich, Roche Glycart AG, Wagistrasse 10, 8952 Schlieren, Switzerland, christina.claus@roche.com.