Article Text
Abstract
Background Pancreatic ductal adenocarcinoma (PDAC) is associated with very poor survival, making it the third and fourth leading cause of all cancer-related deaths in the USA and European Union, respectively. The tumor microenvironment (TME) in PDAC is highly immunosuppressive and desmoplastic, which could explain the limited therapeutic effect of immunotherapy in PDAC. One of the key molecules that contributes to immunosuppression and fibrosis is transforming growth factor-β (TGFβ). The aim of this study was to target the immunosuppressive and fibrotic TME in PDAC using a novel immune modulatory vaccine with TGFβ-derived peptides in a murine model of pancreatic cancer.
Methods C57BL/6 mice were subcutaneously inoculated with Pan02 PDAC cells. Mice were treated with TGFβ1-derived peptides (major histocompatibility complex (MHC)-I and MHC-II-restricted) adjuvanted with Montanide ISA 51VG. The presence of treatment-induced TGFβ-specific T cells was assessed by ELISpot (enzyme-linked immunospot). Changes in the immune infiltration and gene expression profile in tumor samples were characterized by flow cytometry, reverse transcription-quantitative PCR (RT-qPCR), and bulk RNA sequencing.
Results Treatment with immunogenic TGFβ-derived peptides was safe and controlled tumor growth in Pan02 tumor-bearing mice. Enlargement of tumor-draining lymph nodes in vaccinated mice positively correlated to the control of tumor growth. Analysis of immune infiltration and gene expression in Pan02 tumors revealed that TGFβ-derived peptide vaccine increased the infiltration of CD8+ T cells and the intratumoral M1/M2 macrophage ratio, it increased the expression of genes involved in immune activation and immune response to tumors, and it reduced the expression of myofibroblast-like cancer-associated fibroblast (CAF)-related genes and genes encoding fibroblast-derived collagens. Finally, we confirmed that TGFβ-derived peptide vaccine actively modulated the TME, as the ability of T cells to proliferate was restored when exposed to tumor-conditioned media from vaccinated mice compared with media from untreated mice.
Conclusion This study demonstrates the antitumor activity of TGFβ-derived multipeptide vaccination in a murine tumor model of PDAC. The data suggest that the vaccine targets immunosuppression and fibrosis in the TME by polarizing the cellular composition towards a more pro-inflammatory phenotype. Our findings support the feasibility and potential of TGFβ-derived peptide vaccination as a novel immunotherapeutic approach to target immunosuppression in the TME.
- Immunogenicity, Vaccine
- Immunotherapy
- Tumor Microenvironment
- Vaccination
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Transforming growth factor-β (TGFβ) is a key molecule involved in immunosuppression and fibrosis. Circulating TGFβ-specific T cells that recognize cells in a TGFβ-dependent manner have been described in patients with cancer.
WHAT THIS STUDY ADDS
Here we show that a TGFβ-derived multipeptide vaccination can control tumor growth in a murine model of pancreatic cancer by reducing fibrosis and by generating a pro-inflammatory tumor microenvironment.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These findings support the therapeutic potential of TGFβ-derived peptide vaccination as a novel immunotherapeutic approach to target immunosuppression and fibrosis in pancreatic cancer.
Introduction
Pancreatic cancer is the seventh leading cause of cancer-related death globally, ranking third in the USA and fourth in the European Union.1 2 Pancreatic ductal adenocarcinoma (PDAC) is the most common form of pancreatic cancer and accounts for 90% of the reported cases.1 Most patients have metastatic PDAC at the time of diagnosis, limiting surgery as a potential curative intervention and resulting in an overall 5-year survival rate of 5%.3 The poor survival rate can be attributed to late diagnosis due to the lack of disease-specific symptoms, early metastasis and the lack of effective treatment for non-resectable patients.4 Although the clinical benefit of nivolumab and ipilimumab in combination with radiotherapy in patients with refractory metastatic pancreatic cancer has been recently reported in a phase II trial,5 immunotherapy still shows limited efficacy in most patients with PDAC.6 This resistance can be attributed to the intrinsic immunosuppressive, non-immunogenic and desmoplastic nature of pancreatic tumors.6 PDAC is characterized by low T-cell infiltration and a high abundance of suppressive cells, including regulatory T cells (Treg), tumor-associated macrophages (TAMs), and cancer-associated fibroblasts (CAFs).7
Transforming growth factor β (TGFβ) is a major promoter of immunosuppression and is highly secreted in the tumor microenvironment (TME) by cancer cells, fibroblast, macrophages and Tregs.8 Its suppressive effects, including the inhibition of effector T cells, are well documented and have been confirmed in murine models of PDAC.9 In addition, TGFβ is a key player in the development of fibrosis and desmoplastic stroma in PDAC.10 For instance, it polarizes CAFs into myofibroblastic CAFs (myCAFs), a subset of fibroblasts characterized by a significant contribution to extracellular matrix (ECM) deposition,10 which has been linked to immune evasion and failure of cancer immunotherapy.11
Immune modulatory vaccines, which combat immunosuppression by targeting cells in the tumor that express suppressive molecules, offer an appealing and novel approach to cancer immunotherapy. In the last decade, we have described self-reactive, pro-inflammatory T cells, known as anti-Tregs, that specifically target immunosuppressive cells and hinder counter-regulatory feedback signals, particularly in patients with cancer.12 13 Anti-Tregs recognize major histocompatibility complex (MHA)-restricted epitopes derived from proteins expressed by regulatory immune cells, including indoleamine 2,3-dioxygenase (IDO), programmed death-ligand 1 (PD-L1), arginase-1, arginase-2, and galectin-3.14–19 The clinical potential of immune modulatory cancer vaccines that activate anti-Tregs has been shown in murine models of cancer18–21 and in a recent clinical trial conducted at our center, where an impressive response rate was achieved in metastatic melanoma with an immune modulatory vaccine against IDO/PD-L1 in combination with nivolumab in a phase 1/2 trial.22
We recently described the presence of TGFβ-specific T cells in the blood of healthy donors and patients with cancer, and the ability of these cells to recognize and kill cancer cells in a TGFβ-dependent manner.23 24 In this study, we evaluated the efficacy of activating TGFβ-specific T cells by TGFβ-derived peptide vaccination to target immunosuppression and fibrosis in the TME in Pan02, a syngeneic murine model of PDAC. Our data show that TGFβ-derived multipeptide vaccination can control Pan02 tumor growth by polarizing the TME from a suppressive and fibrotic phenotype to a pro-inflammatory niche. We report that treatment with a TGFβ-derived peptide vaccine reduces the TGFβ-signature in the tumor and the infiltration of suppressive cells; increases the frequency of pro-inflammatory immune subsets, generates a pro-inflammatory environment that does not restrain T-cell proliferation, and reduces the expression of genes related to both, ECM-remodeling CAFs (myofibroblasts) and fibroblast-derived collagens. These findings support the therapeutic potential of TGFβ-derived peptide vaccine as a novel immunotherapeutic approach to target immunosuppression and fibrosis in PDAC.
Methods
Mice
Animal experiments were performed at the animal facility of the Department of Oncology, Copenhagen University Hospital, Herlev, Denmark. Female C57BL/6 mice (8–18 weeks old) were bred in-house from a C57BL/6JBomTac background. Experimental procedures were conducted according to Federation of European Laboratory Animal Science Association (FELASA) guidelines and under licenses issued by the Danish Animal Experimentation Inspectorate.
Peptides
Murine TGFβ1-derived T cell epitopes were predicted using NetMHC V.4.0 and NetMHCII V.2.325 for MHC-I and MHC-II-restricted peptides, respectively. Five peptides were selected, one major histocompatibility complex (MHC)-II (H2-Ab)-predicted peptide: mTGFβ (mTGFβ)−18–32 (15mer, LLVLTPGRPAAGLST) and four MHC-I (H2-Kb)- predicted peptides: mTGFβ−4–11 (8mer, SGLRLLPL), mTGFβ−215–223 (9mer, QGFRFSAHC), mTGFβ−282–289 (8mer, TNYCFSST) and mTGFβ−334–342 (9mer, TQYSKVLAL). Peptides were purchased from Schäfer (purity of >90%). mTGFβ−18–32 peptide was reconstituted in 2 mM H2O. The remaining peptides were reconstituted in 20 mM dimethyl sulfoxide (DMSO).
Cell lines
Pan02 was retrieved from the cell line biobank at the National Center for Cancer Immune Therapy (Denmark) and cultured in RPMI-1640 GlutaMAX (Gibco), 10% heat-inactivated fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin (P/S, Gibco). Cell lines were mycoplasma-free, assessed by PCR.
Mouse tumor models
Mice were injected subcutaneously (s.c.) on the right flank with 5×105 Pan02 cancer cells in 100 µL of RPMI-1640. When tumors became palpable, mice were divided into treatment groups by stratified randomization on tumor volume. Tumor length and width were measured three times a week with a digital caliper. Tumor volume was calculated as 0.5 × length × width2. The experimental endpoint was defined by the presence of early signs of tumorous ulceration. Survival could not be assessed due to tumorous ulceration.
Vaccination
Mice were vaccinated s.c. at the base of the tail with two emulsions, one containing 100 µg of the murine TGFβ1-derived MHC-II-restricted peptide (mTGFβ−18–32) and the other containing 50 µg of each MHC-I-restricted peptide (mTGFβ−4–11, mTGFβ−215–223, mTGFβ−282–289 and mTGFβ−334–342), unless otherwise stated in the figure legends. This treatment is referred to as ‘TGFβ vaccine’. Mice were vaccinated on randomization day and 7 days after, unless otherwise stated. The emulsions were generated by mixing the peptide solution with Montanide ISA 51 VG (Seppic) at a 1:1 ratio.
Organ collection and processing
Mice were sacrificed by cervical dislocation. Right inguinal lymph nodes, representing tumor-draining lymph nodes, were harvested and weighed. Spleens were collected. Spleens and lymph nodes were processed through a 70 µm cell strainer and red blood cells were lysed using RBC Lysis Buffer (QIAGEN). Tumors were collected, cut into smaller pieces and digested in RPMI-1640 supplemented with 1% P/S, 2.1 mg/mL collagenase type I (Worthington), 75 µg/mL DNase I (Worthington), and 5 mM CaCl2 for 30 min at 37°C and 300 rpm. Tumors were processed through a 70 µm cell strainer.
Cell sorting
Splenic CD4+ and CD8+ T cells were isolated by positive selection using mouse CD4 (L3T4) MicroBeads and mouse CD8a (Ly-2) MicroBeads (Miltenyi Biotec), according to manufacturer’s instruction. CD45+ and CD45− cells were isolated from Pan02 tumors using mouse CD45 MicroBeads (Miltenyi Biotec), following manufacturer’s instruction.
Generation of bone marrow-derived dendritic cells and macrophages
Femurs and tibias were harvested. Bone marrow cells were collected by flushing the bones with PBS. For the generation of bone marrow-derived dendritic cells (BMDC), 2×106 bone marrow-derived cells were cultured in 10 mL of RPMI-1640 with 10% FBS, 1% P/S and 20 ng/mL murine GM-CSF (PeproTech) in 10 cm Petri dishes (Sigma-Aldrich) and incubated at 37°C. On day 3, cells were supplemented with 10 mL of medium containing 20 ng/mL GM-CSF. On day 6, BMDC was harvested. For the generation of bone marrow-derived macrophages (BMDM), 4×106 bone marrow-derived cells were cultured in 10 mL of DMEM with 10% FBS, 1% P/S and 20 ng/mL murine M-CSF (PeproTech) in 10 cm Petri dishes (Sigma-Aldrich) and incubated at 37°C. On day 2, cells were supplemented with 4 mL of medium containing 20 ng/mL M-CSF. On day 4, media was changed and 10 mL of fresh media with 20 ng/mL murine M-CSF and 20 ng/mL human IL-4 (PrepoTech) was added per dish to polarize BMDM to an M2-like phenotype. On day 6, M2-like BMDM were harvested.
ELISpot
Enzyme-linked immunospot (ELISpot) was performed as described by Bendtsen et al.19 The 8×105 splenocytes, 4×105 cells derived from the lymph node or 2–3×105 CD4+ or CD8+-sorted T cells in 200 µL of RPMI-1640 supplemented with 10% FBS and 1% P/S were added per well in duplicates or triplicates. For experiments with sorted T cells, BMDC were added at a 1:2 ratio (BMDC:T cell). To assess the general response to the TGFβ vaccine, cells were stimulated with a peptide pool consisting of all five peptides included in the TGFβ vaccine to reach a working concentration of 5 µM/peptide. Specific responses are reported as the difference between average number of spots in peptide-stimulated wells and unstimulated wells.
Flow cytometry
The following antibodies/dies (purchased from BioLegend unless otherwise stated) were used for flow cytometry: CD45-PE-Cy7, CD31-FITC, FAP-biotin (R&D system), streptavidin-APC, CD90-BV605, PDPN-APC, Ly6C-AF700, CD26-PerCP-Cy5.5 (eBioscience), αSMA-Cy3 (Sigma), CD11b-PE-Cy7, CD11b-Pacific Blue, F4/80-APC, F4/80-FITC, MR-PE, MR-PE-Cy7, Ly6C-PerCP-Cy5.5, Ly6G-APC-Cy7, Arg1-PE (R&D system), PDL1-APC (BD Biosciences), CD45-FITC, CD3-AF700, CD4-BV421, CD8-BV605 (BD Biosciences), CD25-PE-Cy7, FoxP3-APC and carboxyfluorescein succinimidyl ester (CFSE, Sigma Aldrich). Viability was assessed with Zombie Aqua (BioLegend). Samples were Fc receptor-blocked using mouse FcR blocking reagent (1:10; Miltinyi Biotec). For intracellular staining, samples were fixated and permeabilized with eBioscience Fixation/Permeabilization Concentrate, Diluent and 10X Buffer (Invitrogen). Data were acquired on FACSCanto II (BD Biosciences) or ACEA NovoCyte Quanteon (Agilent) and analyzed with FlowJo V.10.6.1 (Tree Star). Gating strategies can be found in online supplemental figures 8–13.
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Total RNA extraction
Tumors and lymph nodes (≤20 mg) were stored in RNAlater (Invitrogen) at −80°C. For RNA extraction, tumor fragments were transferred to RLT buffer (QIAGEN) and mechanically homogenized with a Tissue Lyser (QIAGEN). RNA was extracted with RNEasy Plus Mini Kit (QIAGEN), following manufacturer’s instructions. RNA concentration was measured with NanoDrop 2000 Spectrophotometer (Thermo Scientific).
RT-qPCR
Reverse transcription of 1 µg of total RNA was done with the iScript cDNA synthesis kit (Bio-Rad), following manufacturer’s instructions. Complementary DNA (cDNA) was diluted 1:3. Reverse transcription-quantitative PCR (RT-qPCR) was performed in technical triplicates on a thermocycler instrument (Roche LightCycler 480) using LightCycler 480 Probes Master (Roche Diagnostics) and the following TaqMan gene expressions assay probes (Life Technologies): Cd3e (Mm01179194_m1), Tgfb1 (Mm01178820_m1), Fap (Mm01329177_m1), S1004a (Mm00803371_m1), Acta2 (Mm01546133_m1), Pdgfra (Mm00440701_m1), Pdgfrb (Mm00435546_m1) and Hprt1 (Mm00446968_m1). Data were normalized to the expression level of Hprt1 (housekeeping gene) and analyzed with the 2−dCT method.
RNA sequencing
RNA sequencing (RNAseq) was performed as previously described26 on tumors from four untreated and three vaccinated mice. In short, 500 ng purified RNA (RNA Integrity Number (RIN) score >7) was enriched for polyadenylated messenger RNA followed by fragmentation, random-primed cDNA synthesis (NEBNext), PCR-mediated indexing (NEBNext), size selection and quantification (KAPA, Roche). The cDNA libraries were sequenced using Illumina NovaSeq 6000. Alignment to GRCm39 and quantification of reads was performed as previously described26 using STAR (V.2.7.8), featureCounts (V.1.6.4), and Ensembl gene transcripts (V.104). Differential gene expression was analyzed by the DESeq2 package (V.1.30, cut-off p adjusted value<0.05 and absolute log2 fold change >0.585). RNAseq data is available on GEO repository (GSE206764). Volcano plots were generated with EnhancedVolcano R package (V.1.8.0). The list of genes used for the generation of heatmaps can be found in online supplemental table 3. All gene lists were obtained from nanoString panel gene lists, except for the cytokine and chemokine gene list, which was self-generated; the pro-fibrotic fibroblast and inflammatory fibroblast gene lists, which were retrieved as per a study by Ledoult et al27 (gene lists named ‘TGFß1_upg’ and ‘TNFα_upg’); and the fibroblast-derived collagen gene list, which was retrieved as per a study by Nissen et al.28 Heatmaps were generated with pheatmap R package (.1.0.12). RNAseq counts were VST (variance stabilizing transformation)-normalized and row mean centered (z-score). Columns represent individual mice and rows represent either the z-score calculated for a specific gene or the mean expression (z-score) across all genes in each gene list, as indicated in the figure legends. Gene Ontology analysis for biological processes were performed using The Gene Ontology Resource software (http://geneontology.org/) using differently upregulated genes as an input. The most specific subclasses according to hierarchy were selected and classified as immune or non-immune processes. Immune-related processes were further classified into 10 different categories: T cell, antigen presentation, cytotoxicity, cytokine, leukocyte, B cell, chemotaxis, innate, neuroimmunity or pathogen immunity-related processes.
Supplemental material
ImmuCC
The computational framework of the CIBERSORT analytical tool29 and the developed ImmuCC signature matrix (511 genes, non-tissue specific)30 suitable for the deconvolution of mouse bulk RNAseq data, were used to characterize and quantify 25 immune cell subtypes. For this study, two population schemes (compact and extended) were defined, resulting in the aggregation of some of the 25 immune subpopulations (online supplemental table 4). The signature matrix was available as an online supplemental table in30. The CIBERSORT software source code in R was obtained from the website: https://cibersort.stanford.edu/, after registration and request for access and download.
Supplemental material
Cytokine measurements
Tumors were harvested and processed as previously described. A total of 0.1×106 cells were added per well to a 96-well plate and incubated for 48 hours at 37°C in RPMI-1640 with 10% FBS and 1% P/S. Cell culture supernatants, named tumor-conditioned media (TCM), were harvested. The concentration of TGFβ1 in TCM was quantified using Bio-Plex Pro TGF-β1 Set (Bio-Rad), following manufacturer’s instruction. Samples were acquired on Bio-Plex 200 system and analyzed with Bio-Plex Manager V.6.
Assays with TCM
The spleen of a tumor-free, untreated mouse was harvested and processed as previously described. 100 uL of a cell suspension containing 0.1×106 CFSE-labeled splenocytes were added per well to a 96-well plate. 100 uL of TCM were added per well. Proliferation was stimulated by adding Dynabeads Mouse T-Activator CD3/CD28 (Gibco, 1:1 bead-to-cell ratio). After a 48 hour-incubation, cells were harvested and stained with Zombie Aqua and CD3-AF700 for flow cytometric analysis. Proliferation index was calculated using FlowJo V.10.6.1 (Tree Star). 100 uL of a cell suspension containing 0.1×106 BMDM polarized towards an M2-like phenotype were added per well to a 96-well plate. 100 uL of TCM were added per well. After a 24 hour-incubation, cells were harvested and stained with F4/80-FITC, CD11b-Pacific Blue, MR-PE-Cy7, Arg1-PE and PD-L1-APC for cytometric analysis.
Data representation
Data was visualized using GraphPad Prism (V.8) or ggplot2 R package (V.3.3.5).
Statistical analysis
Analysis of tumor growth curves was performed using TumGrowth software31 (https://kroemerlab.shinyapps.io/TumGrowth) with default settings and with Bonferroni adjustment for correction for multiple comparison. Linear regression was performed with stats package (V.3.6.2) in R (V.4.0.3) and R Studio (V.1.2.5001). Statistical analyses for the comparison between treatments groups for tumor volume and tumor weight at endpoint, ELISpot responses, flow cytometry analyses, RT-qPCR analyses and RNAseq-derived normalized read counts were performed by an unpaired, two-tailed t test using the rstatix R package (V.0.7.0). Analyses were performed with R (V.4.0.3) and R Studio (V.1.2.5001) software. *p<0.05; **p<0.01; ***p<0.001.
Results
Vaccination with TGFβ-derived peptides activates and expands TGFβ-specific T cells in vivo
To identify immunogenic peptides within the murine TGFβ1 protein sequence, we used NetMHC V.4.0 and NetMHCII V.2.325 servers to predict MHC-I-restricted and MHC-II-restricted epitopes, respectively. We selected five peptides: one 15-mer peptide (mTGFβ−18–32) and four 8-9mer peptides (mTGFβ−4–11, mTGFβ−215–223, mTGFβ−282–289, and mTGFβ−334–342) predicted to be MHC-II (H2-Ab) and four MHC-I (H2-Kb)-restricted, respectively. To test the immunogenicity of the selected peptides, C57BL/6 mice were vaccinated with the TGFβ-derived peptides using Montanide ISA 51 VG as an adjuvant. The immune response in the spleen was evaluated 7 days post-vaccination by interferon (IFN)γ ELISpot. It was confirmed that all five peptides could induce an immune response upon vaccination (figure 1A,B). Next, we investigated the phenotype of TGFβ-specific T cells induced by the TGFβ-derived peptides in IFNγ ELISpot by sorting CD4+ and CD8+ T cells (online supplemental figure 1) from the splenocytes of vaccinated mice. We confirmed the in silico predictions, as the peptides predicted to bind MHC-I and MHC-II generated CD8+ and CD4+ peptide-specific T cells, respectively (figure 1C). Interestingly, mTGFβ−215–223, mTGFβ−282–289, and mTGFβ−334–342 peptides were also able to induce a CD4+ peptide-specific response.
Supplemental material
Vaccination with TGFβ-derived peptides activates and expands CD8+ and CD4+ TGFβ-specific T cells in vivo. (A) mTGFβ−18–32-specific IFNγ-secreting cells in the spleens of mTGFβ−18–32-vaccinated mice (n=4 mice; left) and representative examples of IFNγ ELISpot responses (right). (B) mTGFβ−4–11, 215–223, 282–289, and 334–342-specific IFNγ-secreting cells in the spleens of mTGFβ−4–11, 215–223, 282–289, and 334–342-vaccinated mice, respectively (n=4 mice per peptide; left) and representative examples of IFNγ ELISpot responses (right). For (A) and (B), mice were vaccinated once, and vaccine-induced responses were assessed 1 week after vaccination. Data are presented as mean±SEM. Dots indicate individual mice. (C) Peptide-specific IFNγ-secreting cells in CD4+ or CD8+ sorted T cells (>93% purity, online supplemental figure 1) from the spleens of mTGFβ−18–32, 4–11, 215–223, 282–289, or 334–342-vaccinated mice, assessed by IFNγ ELISpot. Four mice were vaccinated with all five TGFβ-derived peptides on days 0 and 7. On day 14, mice were sacrificed, spleens harvested and pulled, and CD4+ and CD8+ T cells sorted and set up in an IFNγ ELISpot in co-culture with bone marrow-derived dendritic cells (BMDC) as antigen presenting cells (APC) in a 1:2 ratio (T cell:APC). Bars represent mean±SEM. Dots represent technical replicates. ELISpot, enzyme-linked immunospot; IFN, interferon; MHC, major histocompatibility complex; TGFβ, transforming growth factor-β.
TGFβ-derived peptide vaccination induces antitumor immunity in a murine tumor model of pancreatic cancer
We evaluated the antitumor activity of TGFβ-derived peptide vaccination in Pan02, a model of PDAC characterized by high expression of TGFβ132 (online supplemental figure 2), high infiltration of immunosuppressive cells such as TAMs, limited tumorous T-cell infiltration,33 and minimal response to immune checkpoint blockade.34 We assessed the effect of a vaccine containing the TGFβ1-derived MHC-II-restricted peptide alone, all four MHC-I-restricted peptides, or a combination of all five peptides on Pan02-tumor bearing C57BL/6 mice. Mice were vaccinated when tumors became palpable (day 10 post-inoculation) and three additional times (days 17, 24, and 35). We observed that a significant delay in tumor growth was only achieved when MHC-I-restricted 8-9mers were combined with the MHC-II-restricted peptide in a multipeptide vaccine (hereafter referred to as ‘TGFβ vaccine’; figure 2A and B and online supplemental figure 3). Next, we examined the immune response generated against all peptides in each treatment group by IFNγ ELISpot. We verified that mice developed a strong and specific immune response towards the peptide(s) with which they were vaccinated (figure 2C).
Supplemental material
Supplemental material
Vaccination with TGFβ-derived peptides delays tumor growth in the Pan02 model of pancreatic cancer. (A) Average Pan02 tumor growth for untreated mice and mice vaccinated with a TGFβ-derived MHC-II-restricted peptide (mTGFβ−18–32, referred to as "CD4 epitoe"), a pool of four 8-9mers predicted to bind MHC-I (mTGFβ−4–11, 215–223, 282–289, and 334–342, referred to as "CD8 epitopes"), or a combination of all five peptides (referred to as ‘TGFβ vaccine’) in a 4-dose regimen. Mice (n=5–8 mice per group) were vaccinated on days 10, 17, 24, and 35, as indicated by the arrows. Data are presented as mean±SEM. (B) Tumor volume at endpoint (day 38) for the tumor study shown in (A). n=5–8 mice per group. Dots represent individual mice. Data are presented as mean±SEM. (C) TGFβ-derived peptide-specific responses in the spleen on day 38 for each treatment group for the tumor study shown in (A) assayed by IFNγ ELISpot. n=5–8 mice per group. Data are presented as mean±SEM. (D) Average Pan02 tumor growth for mice that were either untreated or vaccinated with a TGFβ vaccine consisting of a pool of one TGFβ-derived MHC-II predicted peptide (mTGFβ−18–32) and four MHC-I-restricted peptides (mTGFβ−4–11, 215–223, 282–289, and 334–342) in a 2-dose regimen. Mice (n=7–8 per group) were vaccinated on days 10 and 17, as indicated by the arrows. Data are presented as mean±SEM. Antitumor effect was confirmed in six independent experiments. A representative example is shown. (E) Individual tumor growth for Pan02 tumor-bearing mice shown in (D). n=7–8 mice per group. Curves represent individual mice. Arrows indicate vaccination days. (F) Tumor volume and (G) tumor weight at endpoint (day 35) for the tumor study shown in (D). n=7–8 mice per group. Dots represent individual mice. Data are presented as mean±SEM. (H) Correlation between tumor weight and tumor-draining lymph node (LN) weight at the endpoint in untreated or TGFβ-vaccinated Pan02 tumor-bearing mice. n=10 mice per group. Dots represent individual mice. (I) Correlation between tumor-draining LN weight and the expression of Cd3e relative to the housekeeping gene Hprt1 shown in arbitrary units (a.u.) in the tumor-draining LN at the endpoint in TGFβ-vaccinated Pan02 tumor-bearing mice. n=10 mice per group. Dots represent individual mice. (J) TGFβ vaccine-specific responses in the tumor-draining LN at the endpoint in Pan02 tumor-bearing mice assayed by IFNγ ELISpot. n=6–8 mice per group. Dots represent individual mice. Data are presented as mean±SEM. *p<0.05 and **p<0.01 according to TumGrowth software for (A) and (D); unpaired two-tailed t test for (F),(G) and (J); and linear regression for (H) and (I). ELISpot, enzyme-linked immunospot; IFN, interferon; MHC, major histocompatibility complex; TGFβ, transforming growth factor-β.
Next, we confirmed that the TGFβ vaccine could significantly delay Pan02 tumor growth when limiting the treatment schedule to only two vaccinations, on days 10 and 17 post-inoculation (figure 2D–2E). A significant reduction in tumor volume (figure 2F) and tumor weight (figure 2G) at endpoint was observed when Pan02 tumor-bearing mice were treated with the TGFβ vaccine compared with the untreated group. Interestingly, a significant negative correlation between tumor weight and tumor-draining lymph node weight at endpoint was observed only in the group treated with the TGFβ vaccine (figure 2H). In this group, bigger lymph nodes correlated with higher expression of Cd3e (figure 2I). In addition, a strong vaccine-specific immune response was observed in the tumor-draining lymph nodes in the group treated with the TGFβ vaccine (figure 2J), suggesting that a stronger vaccine-induced immune response is associated with a better control of tumor growth.
We confirmed that the TGFβ vaccine-induced antitumor effect was not a consequence of general immune activation by the adjuvant (Montanide ISA 51 VG), as a significant delay in tumor growth was only observed in TGFβ vaccine-treated Pan02 tumor-bearing mice, whereas no effect on tumor growth was observed in a group of mice that only received Montanide (online supplemental figure 4A). In addition, we concluded that the antitumor effect observed for the multipeptide TGFβ vaccine was only achieved when TGFβ-derived peptides were combined with Montanide (online supplemental figure 4A), as a TGFβ vaccine-specific immune response was only developed when TGFβ-derived peptides were administered in the presence of an adjuvant (online supplemental figure 4B). The TGFβ vaccine was well tolerated, as this treatment did not affect the evolution of body weight with time, compared with untreated Pan02 tumor-bearing mice (online supplemental figure 4C).
Supplemental material
TGFβ-derived peptide vaccination favors the presence of pro-inflammatory immune subsets in the TME and modulates CAF phenotype
To investigate the mechanism of action underlying the antitumor effect of the TGFβ vaccine, changes in the TME induced by the vaccine were characterized by multicolor flow cytometry. The TGFβ vaccine did not alter the fraction of cancer cells in the tumor (figure 3A and online supplemental figure 5A) or the overall infiltration of leukocytes (figure 3B and online supplemental figure 5B). Interestingly, a reduction in the percentage of endothelial cells upon vaccination was observed (figure 3C and online supplemental figure 5B). Regarding the T-cell compartment, although vaccination with TGFβ-derived peptides did not alter the percentage of total T-cell infiltration (figure 3D and online supplemental figure 5C), we observed a significant increase in tumor-infiltrating CD8+ T cells (figure 3E–G) with no change in the percentage of infiltrating CD4+ T cells (figure 3H). This resulted in a significant increase in the CD8+/CD4+ T cell ratio (figure 3I). No changes were detected in the percentage of Tregs among the CD3+ population (figure 3J and online supplemental figure 5D), which led to a significant increase in the CD8+/Treg ratio (figure 3K). When examining changes in the myeloid compartment, we observed that the infiltration of both polymorphonuclear and monocytic myeloid-derived suppressor cells (MDSC) remained unchanged after TGFβ vaccination (online supplemental figure 5E–5G). Interestingly, although TGFβ vaccination did not affect the percentage of tumor-infiltrating macrophages (figure 3L and online supplemental figure 5H), a drastic increase in the M1 population, followed by a marked decrease in M2 infiltration, was observed (figure 3M, N and P). This resulted in a significant increase in the M1/M2 ratio (figure 3O).
Supplemental material
Vaccination with TGFβ-derived peptides increases the tumoral-infiltration of CD8+ T cells and polarizes tumor-associated macrophages from an M2-like to an M1-like phenotype. Pan02 tumor-bearing mice (n=8 per group) were either left untreated or vaccinated with the TGFβ vaccine on days 10 and 17. Tumors were harvested on day 33, pooled in pairs among treatment groups, and analyzed by flow cytometry. Bar plots show (A) cancer cells gated as CD45− CD31−FAP−, (B) leukocytes gated as CD45+CD31−, (C) endothelial cells gated as CD45− CD31+, (D) CD3+ T cells gated as CD45+CD3+, (E) CD8+ T cells gated as CD45+CD3+CD8+CD4−, (H) CD4+ T cells gated as CD45+CD3+ CD8− CD4+, (I) CD8/CD4 ratio calculated by dividing the percentage of CD8+ T cells among CD3+ cells by the percentage of CD4+ T cells among CD3+ cells, (J) Tregs gated as CD45+CD3+ CD8− CD4+ CD25+ FoxP3+, (K) CD8/Treg ratio calculated by dividing the percentage of CD8+ T cells among CD3+ cells by the percentage of Tregs among CD3+ cells, (L) macrophages gated as CD11b+ F4/80+, (M) M1 macrophages gated as CD11b+ F4/80+ mannose receptor (MR)−, (N) M2 macrophages gated as CD11b+ F4/80+ MR+ and (O) M1/M2 ratio calculated by dividing the percentage of M1 macrophages by the percentage of M2 macrophages in the tumor of untreated or mice treated with the TGFβ vaccine. All populations were gated on single live cells. Gating strategy can be found in online supplemental figures 8–10. Data are presented as mean±SEM. Dots represent pooled tumors (n=3–4 per group). (F) and (G) Representative dot plots of CD4+ and CD8+ cells in the CD3+ population of (F) untreated or (G) TGFβ vaccine-treated mice. (P) Representative histograms of MR on macrophages in untreated mice or mice treated with the TGFβ vaccine shown in (M) and (N). ns, not significant; *p<0.05 and **p<0.01 according to unpaired two-tailed t test. TGFβ, transforming growth factor-β; Tregs, regulatory T cells.
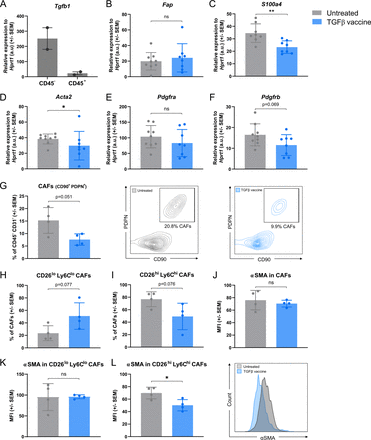
It is well known that TGFβ is highly expressed by CAFs.35 We confirmed that Tgfb1 was mainly expressed in the CD45− compartment of Pan02 tumors from untreated mice (figure 4A), which is mainly composed of cancer cells and CAFs. As TGFβ plays a key role in the recruitment of stromal fibroblasts to the tumor, induces local CAF proliferation and promotes a fibroblast-to-myofibroblast transition,36 we investigated whether vaccination with TGFβ-derived peptides had an impact on the proportion and phenotype of CAFs in Pan02 tumors. We assessed the expression of five CAF biomarkers commonly identified in PDAC37: Fap, S1004a, Acta2, Pdgfra, and Pdgfrb (figure 4B–F) and found that Pan02 tumors from vaccinated mice had significantly lower expression of S1004a (figure 4C) and Acta2 (figure 4D). The same trend was observed for Pdgfrb (figure 4F). We next performed flow cytometric analysis of the CAF compartment and found that the percentage of CAFs (identified as CD90+ PDPN+, as described in a study by Grauel et al38) in Pan02 tumors was reduced upon treatment with the TGFβ vaccine (figure 4G). Grauel et al characterized two subsets of murine CAF according to their CD26 and Ly6C expression.38 When we examined these two CAF subsets in Pan02 tumors, we found that the TGFβ vaccine induced a trend towards an increase in the percentage of CD26lo Ly6Clo CAFs (figure 4H and online supplemental figure 5I), which was followed by a respective trend towards a decrease in the percentage of CD26hi Ly6Chi population (figure 4I and online supplemental figure 5I). As myofibroblasts are characterized by a high expression of Acta2,37 we assessed the expression of αSMA (protein encoded by the Acta2 gene) based on mean fluorescence intensity (MFI) in the defined CAF subsets. Based on MFI, we found that αSMA expression was not affected in CAFs (figure 4J) or CD26lo Ly6Clo CAFs (figure 4K) on vaccination. However, the TGFβ vaccine resulted in a significant reduction in αSMA expression in the CD26hi Ly6Chi CAF population (figure 4L).
TGFβ vaccine reduces the percentage of cancer-associated fibroblasts (CAF) and the expression of Acta2 (αSMA), a classic myofibroblast marker, in Pan02 tumors. (A) Tgfb1 expression relative to the housekeeping gene Hprt1 in the CD45+ and CD45- fractions of tumors from untreated Pan02-tumor bearing mice assessed by RT-qPCR at endpoint. Data are presented as mean±SEM. Dots represent individual mice (n=2 per group). (B)–(F) Expression of CAF-related genes: (B) Fap, (C) S1004a, (D) Acta2, (E) Pdgfra and (F) Pdgfrb in tumors from untreated or TGFβ vaccine-treated Pan02 tumor-bearing mice. Mice were either left untreated or vaccinated with the TGFβ vaccine on days 10 and 17 post-inoculation. Tumors were harvested at endpoint (day 29) and gene expression was assessed by RT-qPCR. Gene expression relative to the housekeeping gene Hprt1 is shown. Data are presented as mean±SEM. Dots represent individual mice (n=8–9 per group). (G)–(L) Flow cytometric analysis of the CAF population in tumors from untreated or TGFβ-vaccinated Pan02 tumor-bearing mice. Pan02-inoculated mice (n=8 per group) were either left untreated or vaccinated with the TGFβ vaccine on days 10 and 17. Tumors were harvested at endpoint (day 29), pooled in pairs among treatment groups, and analyzed by flow cytometry. (G, left) Percentage of CAFs, gated as CD90+ PDPN+, cells across treatment groups. (G, middle) and (G, right) Representative contour plots of CD90+ PDPN+ cells in the CD45− CD31− population shown in (G) of (G, middle) untreated or (G, right) TGFβ vaccine-treated mice. (H) and (I) Percentage of (H) CD26lo Ly6Clo and (I) CD26hi Ly6Chi CAF subsets—as previously described38—across treatment groups. (J)–(L) Mean fluorescence intensity (MFI) of αSMA (x103) in (J) CAFs, (K) CD26lo Ly6Clo CAFs and (L, left) CD26hi Ly6Chi CAFs. (L, right) Representative histograms of αSMA on CD26hi Ly6Chi CAFs in untreated mice or mice treated with the TGFβ vaccine shown in (L, left). All populations were gated on live cells. Gating strategy can be found in online supplemental figure 11. Data are presented as mean±SEM. Dots represent pooled tumors (n=4 per group). a.u., arbitrary units; ns, not significant; *p<0.05, **p<0.01 according to unpaired two-tailed t test. RT-qPCR, reverse transcription-quantitative PCR; TGFβ, transforming growth factor-β.
TGFβ vaccine promotes changes in gene expression in the tumor associated with an increased anti-tumor immune response and a reduced fibrotic environment
Next, we performed bulk RNA sequencing on tumors from Pan02 tumor-bearing mice that were either untreated or treated with the TGFβ vaccine. Differential gene expression analysis identified a total of 545 upregulated and 258 downregulated genes on TGFβ vaccination (figure 5A, online supplemental figure 6A, online supplemental table 1). Gene Ontology analysis for biological processed revealed that 74% of the enriched processes within the upregulated genes were related to immune function. (online supplemental figure 7 and online supplemental table 2). Both innate and adaptive immune-related processes were enriched. Interestingly, ‘CD8+ T-cell activation’, ‘Antigen presentation’, ‘T-cell-mediated cytotoxicity’, and ‘B cell receptor signaling’-related processes were enriched, which is indicative of the development of an antitumor immune response on vaccination (figure 5B). Specifically, a higher expression of Cd8a, Tnfa, Gzmb and Cd69 was observed in the vaccinated group (online supplemental figure 6B). Moreover, tumors from vaccinated mice had increased average expression of a panel of genes involved in antigen presentation, lymphocyte activation, cytokines and chemokines (figure 5C), which indicates that the TGFβ vaccine activates an immune response against the tumor. Tumors from vaccinated mice additionally had increased average expression of genes involved in inflammation, IFN signaling and Toll-like receptor signaling (figure 5C), demonstrating that the TGFβ vaccine can generate a pro-inflammatory TME. Lastly, the TGFβ vaccine resulted in an increased average expression of genes involved in phagocytosis, immune response to tumors and apoptosis (figure 5C), which suggests that the response induced by the vaccine is indeed antitumorigenic. Next, we quantified the infiltration level of different immune cells through RNAseq expression data deconvoluted with the ImmuCC algorithm. The results supported the TGFβ vaccine-induced increase in the CD8/CD4 and CD8/Treg ratios observed by flow cytometry (online supplemental figure 6C). In addition, a trend towards higher infiltration of natural killer cells and B cells on TGFβ vaccination was observed.
Supplemental material
Supplemental material
Supplemental material
Supplemental material
TGFβ vaccine promotes changes in intratumoral gene expression associated with the generation of a pro-inflammatory environment, the development of an antitumor immune response and a shift in the phenotype of CAF from fibrotic to inflammatory. Pan02 tumor-bearing mice were either left untreated or vaccinated with the TGFβ vaccine on days 10 and 17. Tumors were harvested (n=4 for untreated, n=3 for TGFβ vaccine) on day 30, RNA extracted, and bulk RNAseq performed. (A) Volcano plot showing differentially expressed genes (false discovery rate (FDR)<0.05 and absolute log2 fold-change>0.585) in Pan02 tumors from TGFβ-vaccinated mice compared with untreated tumors. n=545 upregulated and n=258 downregulated genes. (B) Gene ontology (GO) analysis for immune-related biological processes associated with significantly upregulated genes in Pan02 tumors from TGFβ-vaccinated mice compared with untreated tumors. Representative processes for each category are shown. Remaining processes can be found in online supplemental table 2. (C) Heatmaps illustrating the average gene expression level of a panel of genes related to antigen presentation, lymphocyte activation, cytokines and chemokines, inflammation, interferon signaling, TLR pathway, phagocytosis, immune response to tumors and apoptosis in Pan02 tumors from untreated (U1–U4) or TGFβ-vaccinated mice (T1–T3). Gene lists can be found in online supplemental table 3. All gene lists were obtained from nanoString panels, except for the cytokine and chemokine gene list, which was self-generated. Columns represent individual mice. RNAseq counts were VST-normalized and row mean centered (z-score). Rows represent the mean expression (mean z-score) across all genes in each gene list. (D–E) Box plots showing the expression level assessed by RNAseq and presented as VST-normalized counts of (D) genes encoding ligands, receptors, and molecules involved in TGFβ signaling, assembly, or activation, and (E) myCAF-related genes38 in Pan02 tumors from untreated or TGFβ-vaccinated mice. Dots represent individual mice. *p<0.05, **p<0.01 according to unpaired two-tailed t test. (F) Heatmap illustrating the gene expression level of a panel of 15 fibroblast-derived collagen genes in Pan02 tumors from untreated (U1–U4) or TGFβ-vaccinated mice (T1–T3). Gene lists were obtained as per a study by Nissen et al28 and can be found in online supplemental table 3. Rows represent individual genes and columns represent individual mice. RNAseq counts were VST-normalized, log2 transformed, and row mean centered (z-score). *differentially expressed genes identified by DESseq2 (FDR<0.05 and absolute log2 fold-change >0.585). (G) Heatmaps illustrating the average gene expression level of a panel of genes representative of a pro-fibrotic fibroblast phenotype and an inflammatory fibroblast phenotype in Pan02 tumors from untreated (U1–U4) or TGFβ-vaccinated mice (T1–T3). Gene lists were obtained as per a study by Ledoult et al27 and can be found in online supplemental table 3. Columns represent individual mice. RNAseq counts were VST-normalized and row mean centered (z-score). Rows represent the mean expression (mean z-score) across all genes in each gene list. CAF, cancer-associated fibroblast; MHC, major histocompatibility complex; myCAF, myofibroblastic CAF; RNAseq, RNA sequencing; TGFβ, transforming growth factor-β; TLR, Toll-like receptor.
We examined the expression of several TGFβ-related genes in the tumor and found that TGFβ ligands and receptors did not have distinctive alterations in the expression patterns upon TGFβ vaccination (figure 5D, top). When assessing the expression of genes coding for proteins involved in canonical TGFβ downstream signaling, we found that TGFβ vaccination significantly reduced the expression of Smad3, suggesting reduced TGFβ signaling in tumors from vaccinated mice (figure 5D, bottom). Interestingly, in the vaccinated group, we also observed a reduction in the expression of genes encoding latent transforming growth factor beta binding protein 1 (LTBP1; Ltbp1), a TGFβ-binding protein required for the structural assembly and secretion of TGFβ, and thrombospondin-1 (THBS1; Thbs1), a major regulator of TGFβ activation (figure 5D, bottom). In line with the significant reduction in endothelial cells in tumors from mice treated with the TGFβ vaccine (figure 3C), we observed a tendency towards reduced expression of Vegfa and Flt1, genes encoding vascular endothelial growth factor A (VEGFA) and vascular endothelial growth factor receptor 1 (VEGFR1), respectively, in tumors from vaccinated mice (online supplemental figure 6D).
Using single-cell RNAseq, Grauel et al defined murine myofibroblasts (myCAFs) as a subset of Acta2+CAF subset characterized by a higher expression of genes associated with ECM remodeling, including Tagln, Lrrc15, and Mfap4.38 As vaccination with TGFβ-derived peptides resulted in a lower expression of Acta2 and αSMA expression based on MFI in CD26hi Ly6Chi CAFs in Pan02 tumors, we evaluated the effect of the vaccine on the expression of additional myCAFs-related genes reported by Grauel et al.38 RNAseq data showed a significant decrease in the intratumoral expression of Acta2 and Tagln, with a trend towards reduced expression of Lrrc15 on TGFβ vaccination (figure 5E) As myCAFs are characterized by a significant contribution to ECM deposition,10 we assessed whether the reduction in the expression of myCAFs-related genes had an impact on ECM deposition. We found that tumors from vaccinated mice expressed lower levels of fibroblast-derived collagens compared with untreated mice (figure 5F). Interestingly, Col6a1, Col6a2, Col6a3, and Col27a1 came up as differentially downregulated genes (online supplemental table 1). As TGFβ is a key a driver of fibrosis in many conditions, including PDAC,10 we assessed the fibrotic status of Pan02 tumors after treatment with the TGFβ vaccine by exploring the average expression of genes representative of a pro-fibrotic fibroblast phenotype and an inflammatory fibroblast phenotype, as described by Ledoult et al.27 We observed a marked reduction in the overall pro-fibrotic transcriptional program in Pan02 tumors treated with the TGFβ vaccine (figure 5G). In turn, a higher expression of inflammatory fibroblast-related genes upon treatment was found (figure 5G).
TGFβ vaccine reduces immunosuppression of T cells and macrophages in the TME
We assessed whether the generation of a pro-inflammatory environment by TGFβ vaccination would affect the intratumoral secretion profile of soluble immunosuppressive factors, and whether this could have an impact on T-cell function. Tumor-conditioned media (TCM) was generated from untreated or vaccinated mice by culturing Pan02 whole-tumor digests for 48 hours and harvesting the supernatant. We confirmed that TCM generated from mice treated with the TGFβ vaccine had lower concentration of TGFβ1, compared with TCM from untreated mice (figure 6A). We assessed whether this difference in levels of TGFβ1 in TCM would affect T-cell proliferation. While the proliferation of anti-CD3/CD28-stimulated T cells from an untreated tumor-free mouse was strongly impaired when cultured with TCM from untreated mice, the TCM from mice treated with the TGFβ vaccine had a limited impact on T-cell proliferation (figure 6B). We showed that Tgfb1 is mainly expressed by cancer cells and CAFs in Pan02 (figure 4A). However, treatment with the TGFβ vaccine drastically polarized macrophages from an M1-like to an M2-like phenotype (figure 3M–3P). We assessed whether the polarization of macrophages in the treated group could be explained due to an indirect effect of the TGFβ vaccine in this population. To do so, bone marrow-derived macrophages (BMDM) were polarized towards an M2-like phenotype with interleukin (IL)-4 and cultured with TCM generated from untreated or TGFβ vaccinated-mice. We observed that the fraction of M1-like macrophages was increased when M2-like BMDM were cultured with TCM from mice treated with the TGFβ vaccine, compared with TCM from untreated mice, leading to an increase in the M1/M2 ratio (figure 6C). This increase was followed by a reduction in the expression based on MFI of the M2-like markers arginase-1 (Arg1) and PD-L1 (figure 6D–6E).
TGFβ vaccination results in reduced intratumoral secretion of TGFβ and reduces immunosuppression of T cells and macrophages in the tumor microenvironment. Pan02 tumor-bearing mice were either left untreated or vaccinated with the TGFβ vaccine on days 10 and 17. Tumors were harvested (n=4 per group) on day 24 and pooled in pairs among treatment groups. Tumor-conditioned media (TCM) secreted by 0.1×106 cells from the tumor digest during a 48 hours culture was harvested. (A) Quantification of TGFβ1 protein levels in TCM from untreated and TGFβ-vaccinated mice. (B) Effect of TCM from untreated or TGFβ-vaccinated mice on the proliferation of anti-CD3/CD28-stimualted naïve splenocytes from an untreated tumor-free mouse. Naïve splenocytes were CFSE-labeled and activated with Dynabeads Mouse T-Activator CD3/CD28 (1:1 ratio) for 48 hours in the presence or absence of TCM generated from tumors from untreated or TGFβ-vaccinated mice. Proliferation of live CD3+ cells was measured by flow cytometry. Gating strategy can be found in online supplemental figure 12. (B, left) Proliferation index in CD3+ cells across culture conditions. (B, right) Representative histograms of CFSE staining in live CD3+ cells (B). (C–E) Effect of TCM from untreated or TGFβ-vaccinated mice on the phenotype of bone-marrow derived macrophages (BMDM) from an untreated tumor-free mouse that were polarized with IL-4 to an M2-like phenotype. BMDM were cultured for 24 hours in the presence of TCM generated from tumors from untreated or TGFβ-vaccinated mice. The phenotype of BMDM was assessed by flow cytometry. (C, top) M1/M2 ratio in BMDM cultured with TCM generated from tumors from untreated or TGFβ-vaccinated mice. Macrophages were gated as CD11b+ F4/80+, M1 and M2 macrophages were gated as CD11b+ F4/80+ mannose receptor (MR)− and CD11b+ F4/80+ MR+, respectively. Gating strategy can be found in online supplemental figure 13. (C, bottom) Representative histograms of MR on macrophages across culture conditions shown in (C, top). (D, top) Mean fluorescence intensity (MFI) of Arg1 macrophages cultured with TCM generated from tumors from untreated or TGFβ-vaccinated mice. (D, bottom) Representative histograms of Arg1 on macrophages across culture conditions shown in (D, top). (E, top) MFI of PD-L1 macrophages cultured with TCM generated from tumors from untreated or TGFβ-vaccinated mice. (E, bottom) Representative histograms of PD-L1 on macrophages across culture conditions shown in (E, top). Bar plots are presented as mean±SD. Dots represent data derived from TCM generated from pooled tumors (n=2 per group). Arg1, arginase-1; IL, interleukin; PD-L1, programmed death-ligand 1; TGFβ, transforming growth factor-β.
Discussion
Immunotherapy has yielded limited efficacy in PDAC, one reason being the intrinsic immunosuppressive, non-immunogenic, and fibrotic nature of pancreatic tumors.6 Here, we describe TGFβ, a key promoter of both immunosuppression and desmoplasia,9 as a novel target for immune modulatory vaccines that can combat both immunosuppression and fibrosis in the TME.
Increasing evidence emphasizes the importance of combining MHC-I and MCH-II-restricted T-cell epitopes in cancer vaccines for a successful clinical outcome due to the crucial role of CD4+ T cells in the development of effective antitumor immunity.39 We demonstrated that stabilization of Pan02 tumor growth could only be achieved when TGFβ1-derived MHC-I and MCH-II-restricted T-cell epitopes are combined, stressing the importance of CD4+ T cells in an immune modulatory vaccine setting. Interestingly, we showed that 8-9mers TGFβ1-derived peptides could elicit not only a CD8+ but also a CD4+ specific response. The ability of short peptides to activate a specific CD4+ response has been previously reported.40
In our study, we show that the TGFβ vaccine did not alter the expression of genes encoding TGFβ isoforms or TGFβ receptors. However, at the protein level, we observed that tumors from TGFβ-vaccinated mice secreted less active TGFβ1. We found that the TGFβ vaccine reduced the tumorous expression of Smad3, which may be indicative of lower TGFβ-canonical signaling activity in these tumors. This reduction was accompanied by lower expression of Ltbp1 and Thbs1 upon vaccination. LTBP1 is essential for the correct assembly of TGFβ prior to its secretion.41 Therefore, reduced expression of Ltbp1 may result in lower secretion of TGFβ complexes. In addition, THBS1 is one of the most widely studied mechanisms of latent TGFβ activation.42 Thus, we propose that lower transcript levels of Thbs1 contribute to the reduced intratumoral TGFβ1 secretion. We recently reported that TGFβ-specific T cells are able to recognize and kill cancer cells in a TGFβ-dependent manner.23 24 Taken together, these data suggest that lower TGFβ-related activity can be achieved in the tumor by targeting and modulating TGFβ-expressing cells with TGFβ-derived peptide vaccination.
The results of this study show that TGFβ vaccination can turn the non-immunogenic Pan02 tumor into a pro-inflammatory environment, as illustrated by higher CD8+ T-cell infiltration or the shift in the TAM phenotype from an M2, suppressive-like phenotype to an M1, pro-inflammatory phenotype in tumors from vaccinated mice. It has been shown that higher CD8+ T-cell infiltration and lower intratumoral expression of the M2 markers, CD163 and mannose receptor, correlate with increased disease-free survival (DFS) and overall survival (OS) in patients with PDAC,43 44 supporting the importance of these findings. We demonstrated that TCM from mice treated with the TGFβ vaccine can reduce the expression of M2-like markers such as mannose receptor, Arg1 and PD-L1 in M2-like macrophages, compared to TCM from untreated mice. This suggests that the modulation of the phenotype of macrophages by the TGFβ vaccine might be, at least in part, an indirect effect of TGFβ-specific T cells. Transcriptomic analyses of Pan02 tumors from mice that received the TGFβ vaccine confirmed that the treatment generates a pro-inflammatory microenvironment, which can favor the development and maintenance of an antitumor immune response. In addition, we confirmed that the TGFβ vaccine can reduce immunosuppression in the TME, as exposure to TCM from vaccinated mice, compared with TCM from untreated mice, restored the ability of T cells to proliferate and increased the M1/M2 ratio in vitro. These results are in line with the mechanism of action suggested for immune modulatory vaccines: the repolarization of the TME from a suppressive niche into a pro-inflammatory environment where anti-tumor responses can occur.14
CAFs are one of the main sources of TGFβ in the TME.35 Grauel et al reported that genes encoding TGFβ proteins are predominantly expressed by myCAFs, compared with other CAF subsets.38 We have shown that vaccination with TGFβ-derived peptides not only reduces the percentage of CAFs in the tumor of Pan02 tumor-bearing mice but also the expression of αSMA based on MFI in a specific CAF subset (PDPN+ CD90+ CD26hi Ly6Chi). Gene expression analysis revealed that Pan02 tumors form mice treated with the TGFβ vaccine had a reduced expression of Acta2 and Tagln, two genes highly expressed by myCAFs.38 Transcriptomic analyses of CAF populations revealed that high Acta2-expressing CAFs, myCAFs, contribute to a high extent to ECM remodeling and collagen deposition.45 We found that tumors from vaccinated mice expressed lower levels of fibroblast-derived collagens compared with untreated mice. Taken together, these data suggests that myCAFs could be a direct target for TGFβ-specific T cells. These findings are in line with the work by Grauel et al,38 who reported that TGFβ blockade with a TGFβ-blocking antibody preferentially targeted myCAFs in murine tumor models. In addition, we have shown, at a transcriptional level, that vaccination with TGFβ-derived peptides turns the pro-fibrotic signature of Pan02 tumors into an environment characterized by a higher expression of inflammatory fibroblast-related genes upon treatment, suggesting that the TGFβ vaccine can indeed reduce fibrosis in the desmoplastic TME of PDAC. Desmoplasia in pancreatic cancer results in a physical barrier to immune infiltration.9 In addition, a collagen-rich environment can directly impair T-cell activation and function.46 We hypothesize that the increased tumorous infiltration of CD8+ T cells upon TGFβ vaccination can be explained by the homing of TGFβ-specific T cells to the tumor, together with the remodeling of the desmoplastic TME, which results in a more physically permissive tumor.
We found that ‘B cell receptor signaling pathway’ and ‘immunoglobulin production’ processes were among the biological processes associated with the differentially upregulated genes in tumors from TGFβ-vaccinated mice compared with untreated mice. In human PDAC, tumor-infiltrating B cells are preferentially located within tertiary lymphoid structures (TLSs).47 Therefore, our data might suggest that TGFβ vaccination could enhance the development of TLSs in the tumor, which has been associated with a favorable impact on the OS and DFS of patients with PDAC.47
Patients with PDAC carrying an angiogenic signature have increased expression of genes involved in TGFβ signaling.48 In addition, TGFβ signaling enhances angiogenesis in murine tumor models of PDAC, which can be controlled with TGFβ type I receptor inhibitors.49 In line with these findings, we report that TGFβ-derived peptide vaccination is associated with lower number of cells positive for CD31, an endothelial marker, in the tumor and with a trend towards reduced expression of genes encoding VEGFA and VEGFR1 in the tumor, suggesting that targeting TGFβ-expressing cells might partially suppress neo-angiogenesis.
The pro-tumorigenic effects of TGFβ have spurred the development of several drugs aimed at inhibiting TGFβ, including galunisertib, which has been tested in patients with pancreatic adenocarcinoma and demonstrated some clinical effect.50 An interesting therapeutic combination could be TGFβ inhibition with TGFβ vaccination, as, in this situation, vaccine-activated T cells would be less affected by the immune suppressive activity of TGFβ.
Peptide-based cancer vaccines have been proven to be safe in numerous clinical trials.51 We previously reported that immune modulatory vaccines are also safe.21 For instance, the overall safety of an immune modulatory vaccine against IDO/PD-L1 in combination with nivolumab in a phase 1/2 trial of metastatic melanoma was comparable to nivolumab monotherapy.22 In line with these findings, we show that immune modulatory vaccine with TGFβ-derived peptides is a safe therapeutic approach. Immune checkpoint inhibitors (ICIs) have provided unprecedented clinical success in cancer treatment. However, in the case of PDAC, ICIs as monotherapy does not effectively improve prognosis, partially due to the particularly immunosuppressive TME of PDAC.52 As we described for the immune modulatory IDO/PD-L1-based vaccine,22 combinational strategies including immune modulatory vaccines to target the suppressive TME are needed to enhance the efficacy of ICIs. As a gene signature of TGFβ activation in tumors is linked to a lack of response to ICI therapy,11 we propose a combination treatment based on TGFβ-based immune modulatory vaccine and ICIs to treat PDAC. We hypothesize that the TGFβ vaccine could enhance the efficacy of ICIs by combating immunosuppression in the TME and that, in turn, ICI could promote the antitumor activity of the vaccine by unleashing the inhibition of vaccine-induced TGFβ-specific T cells and other tumor-reactive cells present in the TME. We are currently initiating a phase I trial at the National Center for Cancer Immune Therapy (CCIT-DK) at Copenhagen University Hospital (Herlev, Denmark) to assess the effect of TGFβ-derived peptide vaccination in combination with radiotherapy and ICIs in patients with PDAC.
Conclusions
The TGFβ-based immune modulatory vaccine can control tumor growth in a murine model of PDAC by polarizing the TME from a fibrotic and suppressive phenotype to a pro-inflammatory niche. The TGFβ vaccine reduces the TGFβ-signature in the tumor, it increases the infiltration of tumoral-CD8+ T cells, it polarizes TAM from a suppressive to a pro-inflammatory phenotype, it generates a pro-inflammatory environment that does not restrain T-cell proliferation and reduces the expression of genes related to ECM-remodeling CAFs (myofibroblasts) and fibroblast-derived collagens. These findings support the therapeutic potential of TGFβ-derived peptide vaccine as a novel immunotherapeutic approach to target immunosuppression and fibrosis in PDAC.
Supplemental material
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
We would like to thank Merete Jonassen, Anette Højgaard Andersen and animal caretakers Anne Boye and Ditte Stina Jensen for excellent technical support.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
- Data supplement 2
- Data supplement 3
- Data supplement 4
- Data supplement 5
- Data supplement 6
- Data supplement 7
- Data supplement 8
- Data supplement 9
- Data supplement 10
- Data supplement 11
- Data supplement 12
- Data supplement 13
- Data supplement 14
- Data supplement 15
- Data supplement 16
- Data supplement 17
- Data supplement 18
Footnotes
Twitter @marper2323, @Majkensiers
Contributors Design of the project (MHA), performing experiments (MP-P, SEW-B, AS, MS, MLH, MAJ, IL, LLdlT, SKB, EM), data analysis (MP-P, AS, MOH, LG), interpretation of the data (MP-P, MOH, MHA), providing reagents (DHM, MD, NØ), writing of the manuscript (MP-P and MHA), supervision and guarantor (MHA). All authors read and approved the final manuscript.
Funding This work was supported by Danish Health Authority grant ‘Empowering Cancer Immunotherapy in Denmark’ (grant number 4-1612-236/8), Independent Research Fund Denmark (grant number 0134- 00072B), The Research Council at Herlev and Gentofte Hospital (grant number: N/A), and the following two Danish funds: Fonden til fremme af klinisk cancerforskning (grant number RL WSDOCS.FID1280670) and Tømrermester Jørgen Holm og hustru Elisa f. Hansens Mindelegat (grant number 20039).
Competing interests MHA has made an invention based on the use of transforming growth factor-β-derived peptides for vaccinations. A patent application directed to the invention is owned by the company IO Biotech ApS and lists MHA as the sole inventor. MHA is advisor and shareholder at IO Biotech. IL and EM are employees at IO Biotech. The additional authors declare no competing financial interests.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.