Article Text
Abstract
Background Bispecific antibodies are promising new therapeutics in B cell malignancies. Whether they lead to potent T cell activation despite described T cell dysfunction in chronic lymphocytic leukemia (CLL), and are able to effectively target high-risk or venetoclax-resistant samples, is currently unknown.
Methods CD19+ cell lines or primary (high-risk) CLL were cocultured in vitro with healthy donor (HD) or CLL-derived T cells in the presence of a CD3xCD19 dual affinity retargeting molecule (CD3xCD19 DART). Cell cytotoxicity, T cell activation, proliferation and effector molecule production were analyzed using flow cytometry.
Results Here, we report that a bispecific CD3xCD19 DART mediates efficient killing by HD T cells of CD19+ cell-lines and primary CLL cells, regardless of immunoglobulin heavy chain variable region (IGHV) mutational status TP53 status or chemotherapy, ibrutinib or venetoclax sensitivity. Whereas TCR stimulation of CLL-derived T cells resulted in dysfunctional T cell activation and proliferation, treatment with CD3xCD19 DART led to a similar activation profile in CLL-derived and HD-derived T cells. Consistently, co-culture of CLL derived T cells with JeKo-1 or CLL cells in the presence of CD3xCD19 DART resulted in significant cytotoxicity by both CD4+ and CD8+ T cells. On stimulation of CLL cells with CD40L, CLL cells become resistant to the specific inhibitor of anti-apoptotic Bcl-2 protein venetoclax, due to upregulation of Bcl-2 family members such as Bcl-XL. Nevertheless, CD40L stimulated CLL cells were as efficiently lysed on CD3xCD19 DART treatment as unstimulated CLL cells. Further examination of the mechanism of CD3xCD19 DART mediated killing showed that lysis was dependent on granules, but was independent of BAX/BAK or caspase activity, indicating non-apoptotic cell death.
Conclusions These data show that CD3xCD19 DART in CLL leads to robust T cell activation and lysis of high-risk venetoclax resistant CLL cells through a non-apoptotic mechanism.
- oncology
- immunology
- immunotherapy
- T-Lymphocytes
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplementary information. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Background
In recent years, treatment options for patients with chronic lymphocytic leukemia (CLL) have greatly expanded through the introduction of targeted drugs such as the Bruton’s tyrosine kinase inhibitor ibrutinib and Bcl-2 inhibitor venetoclax (ABT-199). Although these new inhibitors induce durable responses and are generally well tolerated, they are not curative.1 In addition, patients need to remain on therapy continuously, leading to development of resistance, which has already been reported for both inhibitors.2 3 Therefore, additional therapies for CLL are still urgently needed.
Allogeneic hematopoietic stem cell transplantations have shown as a proof-of-principle that long-lasting remissions can occur through a T cell-mediated graft-versus-leukemia effect.4–6 However, allogeneic stem cell transplantations cannot be widely applied in this elderly patient group due to high morbidity and mortality as a result of T-cell mediated graft-versus-host disease.4 6 7 Such adverse events could potentially be circumvented by applying autologous T cell therapies, such as chimeric antigen receptor (CAR) T cells and immune checkpoint blockade (ICB). CAR T cell therapy and ICB are effective in multiple B cell malignancies,8–10 but their efficacy in CLL remains disappointing.11–13 The limited responses of autologous based T cell therapies are likely due to the fact that T cells from CLL patients show a dysfunctional phenotype. CLL-derived T cells have a decreased proliferative and cytotoxic potential on stimulation, as well as impaired synapse formation.14 15 Furthermore, T cells of CLL patients have an exhausted phenotype and are skewed towards a short-lived effector phenotype.14 16–18
A third class of autologous T cell therapy is represented by bispecific antibodies. Through binding to CD3, T cells are redirected, to a tumor cell by targeting of the other arm of the bispecific antibody to a tumor-associated antigen. Compared with the labor intensive and therefore expensive production of patient specific CAR T cells, bispecific antibodies have the advantage that they can be provided as off-the-shelf products. In contrast to CAR T cell therapy, this availability allows for repeated treatment. Therefore, bispecific antibodies are an attractive clinical option besides CAR T cells for CLL patients. Blinatumomab, a CD3xCD19 bispecific T cell engager (BiTE), has been the first bispecific to be approved by the Food and Drug Administration for treatment of patients with relapsed or refractory B cell precursor acute lymphoblastic leukemia.19 20 Despite the well-known T cell dysfunction in CLL, blinatumomab has shown to be effective in vitro against CLL.21 Furthermore, CD3xCD19 bispecifics have been shown to induce immune synapse formation and have the potency to induce cell death in ibrutinib resistant CLL samples.22 However, because of a short half-life of the BiTE construct, patients need to be treated continuously.23 Bispecific dual affinity re-targeting (DART) molecules have increased stability due to both disulphide bonds that link the polypeptide chains and by tethering of the DART to an Fc domain, which could prolong half-life up to 6.7 days.24 Earlier it has been reported that CD3xCD19 DARTs led to T cell activation and tumor depletion of B cell malignancies in vitro.25
Because of the ability of bispecific antibodies to induce synapses in CLL,22 and based on the assumption that T cell-mediated cell death differs from venetoclax mediated lysis (which is dependent on the intrinsic apoptosis pathway), we hypothesized that CD3xCD19 DART treatment overcomes T cell dysfunction and will be effective against high-risk venetoclax-resistant samples.
Here, we demonstrate that CD19+ cell lines as well as high-risk primary CLL cells are efficiently killed by HD-derived and CLL-derived T cells in presence of the CD3xCD19 DART. CD3xCD19 DART treatment resulted in significant killing of both allogeneic and autologous CLL, mediated by both CD4+ and CD8+ cells. Furthermore, upregulation of anti-apoptotic molecules, leading to venetoclax resistance, did not affect CD3xCD19 DART mediated lysis, suggesting that non-apoptotic mechanisms are involved. Together, our data indicate that CD3xCD19 DART could serve as a potential salvation therapy in high-risk or venetoclax resistant CLL.
Materials and methods
Patients and controls
Written informed consent was obtained from all subjects in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood of CLL patients (table 1) or buffy coats of (age-matched) HDs (table 2) from Sanquin Blood Supply (Amsterdam, The Netherlands) using Ficoll-Plaque (VWR). All samples were cryopreserved in liquid nitrogen and CLL samples used had a purity of CD5+CD19+ of at least 85%. CLL patients were selected on their diagnosis (CD5+CD19+CD23+ lymphocytosis), treatment history or chemoresistance.
Patient characteristics
Characteristics of healthy donors
DART molecules
The CD3xCD19 DART (JNJ-64052781, also known as MGD011) molecule was constructed as described.24 The CD3xCD19 DART is composed of anti-CD3 sequence XR32 and anti-CD19 sequence BU12. The control DART molecule, CD3xFITC, in which the variable domain sequences of the antifluorescein mAb 4-4-20 replaces the CD19 arm, was engineered in a similar manner. DART molecules were expressed transiently in CHO-S cells and purified to homogeneity by using protein A.
Culture conditions
CLL cells, 3T3 and 3T40 (expressing CD40L) fibroblasts and Ramos cells were cultured in Iscove’s Modified Dulbecco’s Medium (Thermo Fisher Scientific). JeKo-1 and Daudi cells were cultured in RPMI 1640 medium (Thermo Fisher Scientific). Medium was supplemented with 10% fetal calf serum and 1% penicillin/streptomycin.
Flow cytometry
PBMCs were washed with PBA (phosphate buffered saline, 0.5% BSA and 0.02% sodium azide) and stained using fluorescently labeled antibodies for 20 min on ice. The following antibodies were used: CD3 Alexa Fluor (AF)700 (eBioscience), CD3 allophycocyanin (APC) (eBioscience), CD3 V500 (BD Biosciences), CD4 Phycoerythrin (PE)-Cy7 (BD Biosciences), CD4 AF594 (Biolegend), CD4 Brilliant Violet (BV) 605 (BD Biosciences), CD5 PE (eBioscience), CD8 PerCPCy5.5 (BD Biosciences), CD8 V450 (BD Biosciences), CD8 PE-Cy7 (eBioscience), CD19 APC (BD Biosciences), CD20 FITC (BD Biosciences), CD25 APC (BD Biosciences), CD25 BV786 (BD Biosciences), CD71 FITC (eBioscience), CD107a PE-Cy7 (BD Biosciences), interferon γ (IFNγ) Brilliant Ultraviolet (BUV) 395 (BD Biosciences), tumor necrosis factor-α (TNFα) AF700 (BD Biosciences), Granzyme B AF647 (BD Biosciences), Perforin AF488 (Sony) and cleaved poly ADP-ribose polymerase (PARP) PE (BD Biosciences). To exclude dead cells, Fixable Viability Dye eFluor 780 or Fixable Viability Dye eFluor 506 (Thermo Fisher Scientific) was used according to manufacturer’s instructions. For staining of intracellular cytokines or cleaved PARP, cells were fixed and permeabilized using the Fixation/Permeabilization Solution Kit (BD Biosciences). After antibody staining, samples were washed using PBA and acquired on BD FACS Canto or LSR Fortessa flow cytometer and analyzed with FlowJo v10.
3T3 and 3T40 stimulation
3T3 control or 3T40 fibroblasts were irradiated and plated as described.26 After 2 hours of adherence, CLL cells were added to the 3T3 or 3T40 fibroblasts. After 24–48 hours, CLL cells were removed from the fibroblasts and used as target cells in a cytotoxicity assay (see below). Effective CD40L stimulation of the CLL cells was confirmed by testing resistance of the 3T3 and 3T40 stimulated CLL cells to 10 nM ABT-199 for 24 hours.
Cytotoxicity assay
Cell-lines or primary CLL samples were labeled with cell trace violet (CTV) (Thermo Fisher Scientific) according to manufacturer’s instructions and cocultured with HD PBMCs or CLL derived (autologous) T cells in different effector to target (E:T) ratios. Where indicated, T cells were pretreated for 2 hours with 1 µM concanamycin A (CMA) or dimethyl sulfoxide (DMSO) as a control. The treated T cells were thoroughly washed before using in the coculture. 10 or 20 µM of the pan-caspase inhibitor QVD-OPh (APExBIO) was added to the cocultures where indicated. Viability of the target cells was assessed using TO-PRO-3 (Invitrogen) and MitoTracker Orange (Invitrogen) using Flow Cytometry. Specific lysis of target cells was calculated as (% target cell death in treated sample - % cell death target cells in medium control)/(100 – % cell death target cells in medium control) * 100%. Samples were excluded when cell death in medium controls exceeded 50%.
T cell proliferation and activation
PBMCs from HD or CLL patients were labeled with CTV. PBMCs were incubated in the presence of 100 ng/mL CD3xCD19 DART or stimulated with CD3 (clone 1XE) and CD28 (clone 15E8) antibodies. After 2 days activation was measured and after 4 days proliferation and activation were measured by flow cytometry as described above.
Cytokine production and degranulation
PBMCs from HD or CLL patients were incubated in the presence of 100 ng/mL CD3xCD19 DART or stimulated with CD3 (clone 1XE) and CD28 (clone 15E8) antibodies for 2 days. Brefeldin A (10 ug/mL, Invitrogen), GolgiStop (BD Biosciences) and anti-CD107a PE-Cy7 were added 4–6 hours before assessment of degranulation and cytokine production by flow cytometry as described above.
BAX/BAK knockout JeKo-1
Bcl-2-associated X (BAX) and Bcl-2 homologous antagonist/killer (BAK) knockout (KO) JeKo-1 cells were generated using CRSIPR/Cas9 technology. Details regarding single guide RNAs (sgRNAs) targeting BAK and BAX have been published before.27 Each sgRNA was cloned in the lentiCRISPRv2 puro plasmid, a gift from Brett Stringer (Addgene #98290). Lentivirus was produced and used to infect the cell-lines. After selection with puromycin (Sigma) and single cell cloning, knockouts were confirmed by SDS-PAGE with BAK and BAX antibodies (Cell Signaling).
Statistical analysis
To test for normal distribution a D’Agostino-Pearson test or a Shapiro-Wilk test if n=4, was used. P values were calculated by using two-sided paired or unpaired t tests, Wilcoxon test, Mann-Whitney U test, one-way analysis of variance (followed by Tukey’s post hoc test) or Friedman test (followed by Dunn’s post hoc test). Statistical analysis was performed using Graphpad PRISM V.8.0.2 with significance set at P<0.05.
Results
CD3xCD19 dart mediates HD T cell activation and lysis of CD19+ cell-lines in vitro
CD3xCD19 DART (JNJ-64052781 or MGD011) is an Fc bearing bispecific molecule with an approximate molecular weight of 109 kDa and is composed of an anti CD3 (clone XR32) and anti CD19 domain (BU12).24 Previously it has been shown that this DART is specific for CD19, since CD19- cell lines are not affected.25 To assess whether this DART molecule treatment leads to robust T cell activation, age-matched HD PBMCs were cultured in presence of 100 ng/mL CD3xCD19 DART, and activation was compared with direct TCR stimulation with anti-CD3/CD28 antibodies. Robust T cell proliferation was observed when treating HD PBMCs with the CD3xCD19 DART for 4 days, equal to stimulation with anti-CD3/CD28 antibodies for CD4+ T cells (figure 1A). CD8+ T cells showed increased proliferation after treatment with CD3xCD19 DART (figure 1A). Furthermore, exposure to CD3xCD19 DART led to T cell activation as indicated by upregulation of activation markers CD25 and CD71, the degranulation marker CD107a, production of IFN-γ, TNF-α, perforin, and granzyme B in both CD4+ and CD8+ T cells (figure 1B–G and online supplementary file S1A). Enhanced expression of CD25 and the effector molecules was similar in the CD3xCD19 DART conditions compared with anti-CD3/CD28 stimulation (figure 1B–G). As a proof-of-principle that CD3xCD19 DART induces T cell-mediated killing of CD19+ targets, we cocultured HD T cells with the CD19+ mantle cell lymphoma cell-line JeKo-1, in the presence of increasing concentrations of the functional CD3xCD19 DART or a control DART molecule (CD3xFITC). JeKo-1 was chosen because of its coexpression of CD5 and CD19 and loss of TP53, also a feature of high-risk CLL.28 Exposure of JeKo-1 cells to HD T cells in a 1:1 effector to target ratio in the presence of CD3xCD19 DART caused lysis of target cells already after 24 hours, which was further augmented when increasing CD3xCD19 DART concentrations, prolonging exposure up to 4 days (figure 1H), or increasing E:T ratios (figure 1I). Exposure to the control DART molecule did not result in lysis of the CD19+ targets (figure 1H–I). CD3xCD19 DART mediated lysis of CD19-positive cell-lines was also observed using the Burkitt lymphoma cell-line Daudi, and was confirmed using T cells derived from different donors (online supplementary figure S1B,C).
Supplemental material
HD T cells become activated on CD3xCD19 dart treatment and are able to kill CD19+ cell-lines. (A–G) HD PBMCs were CTV labeled and left unstimulated or cultured in the presence of 100 ng/mL CD3xCD19 DART or anti-CD3/aCD28 antibodies. T cell proliferation (A) was measured after 4 days (n=4). (B–G) after 2 days induction of CD25 (B), degranulation measured by CD107a (C), IFNγ production (D), TNFα production (E), perforin production (F) and granzyme B production (G) were measured by fluorescence-activated cell sorting (FACS) (n=4–8). (H–I) FACS analysis of lysis of CTV labeled JeKo-1 cells cocultured in 1:1 (H) or 4:1 (I) E:T ratios and with different concentrations of the CD3xFITC and CD3xCD19 DART molecules for 24 hours and 4 days (n=2). *P<0.05; **P<0.01. CTV, cell trace violet; DART, dual affinity retargeting; HD, healthy donor; IFNγ, interferon γ; ns, not significant; PBMCs, peripheral blood mononuclear cells; TNFα, tumor necrosis factor-α.
CD3xCD19 DART mediates lysis of primary CLL cells independent of IGHV mutation status, presence of TP53 aberrations or chemotherapy or ibrutinib refractoriness
To determine whether exposure to CD3xCD19 DART also leads to lysis of primary CLL cells, HD T cells were incubated with increasing concentrations of the CD3xCD19 DART or control DART molecule in the presence of primary CLL cells for 24 hours or 4 days. Killing of the primary CLL cells could already be observed after 24 hours in a 1:1 E:T ratio, and was dependent on the concentration of CD3xCD19 DART, the E:T ratio, and duration of exposure (4 days) (figure 2A–B and online supplementary S1D–G). Since the prognosis of CLL patients is dependent on the immunoglobulin heavy chain variable region (IGHV) mutational status, presence of TP53 aberrations and/or whether patients are relapsed or refractory after chemotherapy, we tested whether CD3xCD19 DART could induce lysis in unmutated IGHV, mutated IGHV CLL, cells derived from chemorefractory patients and TP53 mutated and/or 17 p deleted patients. Lysis on CD3xCD19 treatment was observed in all subgroups of patients (figure 2C and online supplementary figure S1H). Although, low numbers, first evidence is available that patients who become refractory on new agents such as ibrutinib are still sensitive to CD3xCD19 mediated lysis (online supplementary figure S1I). Taken together, using HD T cells, primary CLL cells can be efficiently lysed by using CD3xCD19 DART irrespective of IGHV mutation status, TP53 aberrations or sensitivity to chemotherapy.
CD3xCD19 DART mediates lysis of primary CLL cells independent of IGHV mutation status, presence of TP53 aberrations or sensitivity to chemotherapy. (A–C) FACS analysis of lysis of CTV labeled primary CLL cocultured with healthy donor T cells in presence of CD3xCD19 DART. (A–B) Primary CLL cells cocultured in 1:1 (A) or 4:1 (B) E:T ratios and with different concentrations of the CD3xFITC and CD3xCD19 DART molecules for 24 hours and 4 days (n=3) (C) lysis of unmutated IGHV (n=5), mutated IGHV (n=4), chemorefractory (n=6) and TP53 mutated/17 p deleted (n=4) CLL by HD T cells in 4:1 E:T ratio after 4 days. CLL, chronic lymphocytic leukemia; CTV, cell trace violet; DART, dual affinity retargeting; HD, healthy donor; M-CLL, mutated IGHV; U-CLL, mutated IGHV.
CD3xCD19 DART induces proliferation, activation and production of effector molecules in CLL-derived T cells
T cells from CLL patients are known to be dysfunctional with regard to activation and proliferation.14 15 18 To test whether CD3xCD19 DART exposure could overcome this dysfunctional phenotype, CLL PBMCs were cultured in the presence of 100 ng/mL CD3xCD19 DART or anti-CD3/CD28 antibodies. As we and others have previously reported,14 18 TCR stimulation of CLL-derived T cells results in impaired proliferation and activation (as measured by upregulation of CD25 and CD71) compared with age-matched HD T cells (figure 3A,B, online supplementary figure S1J). Remarkably, addition of the CD3xCD19 DART induced robust proliferation in both CD4+ and CD8+ T cells derived from CLL patients (figure 3A), similar to HD T cells. In line with this, exposure to CD3xCD19 DART led to activation of CD4+ and CD8+ CLL derived T cells as indicated by upregulation of activation markers CD25 and CD71, the degranulation marker CD107a, production of IFN-γ, TNF-α, perforin and granzyme B (figure 3B–G and online supplementary S1J). Whereas impaired T cell activation was measured on TCR stimulation with anti-CD3/CD28 antibodies compared with healthy controls, CD3xCD19 DART treatment led to equal or even improved activation of CLL derived T cells (figure 3B, online supplementary figure S1J). Also, production of IFN-γ, TNF-α and perforin on CD3xCD19 DART treatment in HD and CLL-derived T cells was equal (figure 3D–G), yet degranulation was significantly increased in both CD4+ and CD8+ T cells in CD3xCD19 treated CLL derived T cells compared with HD (figure 3C). Interestingly, whereas both CLL and HD CD8+ T cells produce granzyme B on TCR or CD3xCD19 DART, CLL derived CD4+ T cells lack behind compared with HD derived CD4+ T cells (figure 3G). Nevertheless, granzyme B expression increased significantly in CLL derived CD4+ T cells on CD3xCD19 DART treatment compared to anti-CD3/CD28 stimulation (figure 3G). Taken together, while TCR stimulation with anti-CD3/CD28 antibodies results in poor T cell activation, proliferation and degranulation in T cells of CLL patients, treatment with CD3xCD19 DART induces fully functional T cell responses in CLL samples.
CLL-derived T cells proliferate and activate and produce effector molecules on CD3xCD19 DART in exposure. (A–F) Age-matched HD* or CLL PBMCs were CTV labeled and left unstimulated or in the presence of 100 ng/mL CD3xCD19 DART or aCD3/aCD28 antibodies. T cell proliferation (A) was measured after 4 days (n=4). (B–G) After 2 days induction of CD25 (B), degranulation measured by CD107a (C), IFNy production (D), TNFα production (E), perforin production (F) and granzyme B production (G) were measured by FACS (n=4–8). *Data from HD PBMCs equal to data in figure 1A–G. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. CLL, chronic lymphocytic leukemia; CTV, cell trace violet; DART, dual affinity re-targeting; HD, healthy donor; IFNγ, interferon γ; PBMCs, peripheral blood mononuclear cells; TNFα, tumor necrosis factor-α.
CD3xCD19 DART treatment mediates lysis of primary CLL cells by CLL-derived T cells
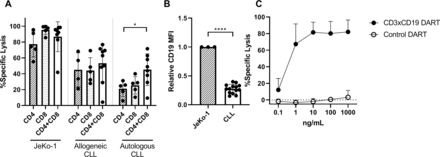
Since it was determined that CD3xCD19 DART exposure leads to enhanced T cell activation, proliferation and production of effector molecules compared with TCR stimulation, we assessed whether T cells of CLL patients are also able to lyse CLL cells. Isolated CD4+ or CD8+ T cells from CLL patients were cocultured separately or mixed (CD4:CD8 ratio of 1:1) with JeKo-1, CLL cells from an allogeneic donor, or autologous CLL cells in the presence of 100 ng/mL CD3xCD19 DART. CLL derived T cells lysed JeKo-1 cells very efficiently (figure 4A). CD4+ T cells alone were equally capable of lysing JeKo-1 or allogeneic CLL cells as CD8+ T cells, and CD4+ and CD8+ together. Although slightly lower compared with lysis of JeKo-1 cells, similar results were found for lysis of allogeneic CLL cells (figure 4A). In the autologous setting, the combination of CD4+ and CD8+ T cells showed more efficient lysis of autologous CLL cells than either subset alone (figure 4A). The more efficient killing of Jeko-1 compared with primary CLL cells might at least in part be explained by higher expression of CD19 on JeKo-1 compared with primary CLL cells (figure 4B). Coculture of CLL T cells (CD4 and CD8 cells combined, CD4:CD8 ratio as present in donor unknown), with primary CLL and increased dosages of CD3xCD19 DART could not improve killing of primary CLL cells, as a plateau in lysis of CLL cells was already reached at 10 ng/mL CD3xCD19 DART (figure 4C). In conclusion, CD3xCD19 DART treatment leads to potent lysis of JeKo-1 cells and (autologous) primary CLL cells mediated by patient-derived T cells.
CLL-derived T cells kill target cells with CD3xCD19 DART in CLL cocultures. (A) Killing of CTV labeled JeKo-1, allogeneic CLL cells or autologous CLL after 4 days of coculture with 100 ng/mL CD3xCD19 DART in a 1:1 E:T ratio with CD4 T cells, CD8 T cells or CD4 and CD8 T cells (CD4:CD8 ratio of 1:1) (n=4–9) (B) relative expression of CD19 of JeKo-1 (n=3) and primary CLL cells (n=14) measured by flow cytometry. (C) killing of CTV labeled CLL after 4 days of coculture with increasing dosages of CD3xCD19 DART in a 1:1 ratio with autologous CLL derived T cells (CD4:CD8 ratio not modified) (n=3). *P<0.05; ****P<0.0001.CLL, chronic lymphocytic leukemia; CTV, cell trace violet; DART, dual affinity retargeting; MFI, mean fluorescence intensity.
CLL cells resistant to venetoclax can still be killed upon treatment with CD3xCD19 DART
The selective Bcl-2 inhibitor venetoclax results in deep responses in blood and bone marrow but seems less effective in eradicating lymph node (LN) residing CLL cells. This might be due to local upregulation of anti-apoptotic molecules such as Bcl-XL and MCL-1.29 We, therefore, assessed effectiveness of CD3xCD19 DART against CLL cells that have acquired an anti-apoptotic profile, after in vitro stimulation by CD40L, which induces upregulation of Bcl-XL and MCL-1, and leads to venetoclax resistance (online supplementary figure S2A).29 On exposure to the CD3xCD19 DART, CD40L-stimulated CLL cells were still effectively lysed by HD T cells (figure 5A). Importantly, CLL cells of a venetoclax-refractory patient could still be lysed on CD3xCD19 DART exposure (online supplementary figure S1I). Furthermore, Bcl-2 overexpressing Ramos cells, which are resistant to apoptotic stimuli such as CD95 or B cell receptor triggering,30 31 were sensitive to CD3xCD19 DART treatment (figure 5B). These results indicate that a block in intrinsic apoptosis does not prevent CD19+ cells from being targeted by CD3xCD19 DART.
Venetoclax-resistant CLL cells can be killed using CD3xCD19 DART. (A) 3T3 or 3T40 treated CLL cells were cocultured for 24 hours with healthy donor T cells in the presence of 100 ng/mL CD3xCD19 dart and assessed for viability (n=10). (B) FACS analysis of lysis of WT Ramos or Ramos-Bcl-2 cells on coculture in 4:1 E:T ratio with HD T cells in presence of control or CD3xCD19 DART for 24 and 48 hours (n=4). (C) Specific lysis of coculture of primary CLL cells with untreated, DMSO treated or CMA treated HD T cells in a 4:1 ratio for 2 days (n=4). (D–E) Viability was assessed of mock transfected (n=1) and BAX/BAK KO clones (n=4) after treatment for 24 hours with (D) 10 μM venetoclax or (E) 100 ng/mL CD3xCD19 or the control dart molecule in 4:1 E:T ratio with HD T cells. (F–G) Specific lysis of primary CLL (F) treated with 100 nM ABT-199 for 24 hours in presence or absence of QVD-OPh (n=6) or (G) cocultured with HD T cells in 4:1 ratio and 100 ng/mL CD3xCD19 dart for 4 days in presence or absence of QVD-OPh (n=6). (H–I) Assessment of cleaved PARP by flow cytometry in (H) JeKo-1 cells cocultured for 24 hours or (I) CLL cells cocultured for 48 hours with HD T cells in a 4:1 ratio in the presence of 100 ng/mL CD3xCD19 DART, with or without 20 μM QVD-OPh (n=4–6). **P<0.01. ABT-199, Bcl-2 inhibitor venetoclax; CLL, chronic lymphocytic leukemia; CTV, cell trace violet; DART, dual affinity retargeting; HD, healthy donor; ns, not significant; WT, wild type.
CMA inhibited CD3xCD19 DART mediated cell death of CLL cells (figure 5C), implying that killing depends on the perforin and granzyme pathway.32 Reported mechanisms of granzyme-mediated lysis of target cells are not conclusive. Initiation of the intrinsic apoptosis pathway through cleavage of Bid, but also direct activation of caspase 3 have been described.33–35 To test dependency of CD3xCD19 DART-mediated cell death on the intrinsic apoptosis pathway, BAX/BAK KO JeKo-1 cell-lines were generated (online supplementary figure S2B). Intrinsic apoptosis blockade was confirmed by resistance to venetoclax treatment after 24 hours (figure 5D). Yet, HD T cells caused similar lysis of the BAX/BAK KO and wild type cell-lines (figure 5E), implying CD3xCD19 mediated cell death does not depend on the intrinsic apoptosis pathway. We next tested involvement of caspases by using the pan-caspase inhibitor QVD-OPh. QVD-OPh rescued venetoclax-induced but not CD3xCD19 DART mediated cell death (figure 5F,G). Similar results were obtained using CD40L stimulated CLL or JeKo-1 as target cells (online supplementary figure S2C–E). These data imply that the mechanism of DART molecule mediated T cell-induced cell death is, at least in part, non-apoptotic. This was further confirmed by the induction of PARP cleavage in JeKo-1 and CLL cells after CD3xCD19 DART exposure, which could be fully blocked by addition of QVD-OPh although the cells still died (figure 5H,I). Taken together, CD3xCD19 DART mediated lysis is dependent on granule exocytosis and can potently induce lysis in venetoclax resistant cells, which seems to be independent of BAX/BAK and caspase activity, and therefore seems non-apoptotic.
Discussion
In this study, we have assessed the effects of a CD3xCD19 targeting DART molecule on CLL in vitro. Our data show that CD19+ cell-lines and primary CLL cells are efficiently lysed by HD T cells using the CD3xCD19 DART. Despite T cell dysfunction in CLL patients after TCR triggering by anti-CD3/CD28 antibodies, CD3xCD19 DART exposure led to efficient T cell stimulation, proliferation and production of effector molecules, and efficient lysis of autologous CLL samples by both CD4+ and CD8+ T cells. Furthermore, (CD40-stimulated) venetoclax resistant CLL samples were efficiently killed on application of the CD3xCD19 DART. The observed cell death was independent of caspase activity, implying that a non-apoptotic activity is involved in CD3xCD19 DART mediated T cell killing.
When comparing CD3xCD19 DART-induced activation of HD T cells to conventional T cell stimulation with anti-CD3/CD28 antibodies in vitro, we could not find any differences in terms of activation markers, degranulation, production of effector molecules and proliferation. In contrast, when comparing these conditions in PBMCs from CLL patients, we found superior T cell function on administration of the CD3xCD19 DART compared with TCR stimulation. CLL T cells have been reported to be deficient in forming immunological synapses on coculture with superantigen pulsed CLL.14 15 However, using a similar CD3xCD19 bispecific construct, CLL derived T cells were readily able to form immunological synapses with target cells already after 15 min of incubation.15 22 The immunological synapse might therefore explain improved T cell function on CD3xCD19 DART treatment, although the exact mechanism remains to be elucidated. T cells receive costimulatory signals at the immunological synapse, which is instrumental for proper T cell activation.36 37 It has been shown that costimulation via CD28 and 4-1BB enhances the efficacy of T cells modified to express an anti-CD19 BiTE, both in vitro and in vivo.38 Since CLL derived T cells are known to be skewed towards a memory/effector phenotype and hence have less T cells expressing CD28, a role for other costimulatory molecules, such as 4-1BB might be an important explanation as to why CLL derived T cells do not fully respond to anti-CD3/CD28 antibodies, but overcome dysfunctional properties when exposed to CD3xCD19 DART. Nevertheless, costimulation provided by unstimulated CLL cells already seem to be enough to elicit proper T cell activation in the CD3xCD19 DART setting, since triggering of CLL cells with CD40L, which is known to upregulate costimulatory molecules, does not further enhance lysis of CLL cells.39
Classically, CD8+ T cells are viewed as the main cytotoxic T cell, but in recent years it has become more clear that CD4+ T cells also have the capability to differentiate into cytotoxic lymphocytes. Cytotoxic CD4+ T cells were mainly observed in chronic viral infections and inflammation, but they have recently also been implied to play a role in antitumor responses.40–43 Here, we demonstrate that CD4+ T cells are also able to induce target lysis on CD3xCD19 DART exposure. Surprisingly, we observed that CLL derived CD4+ T cells produced less granzyme B compared with their HD derived counterpart on stimulation with CD3xCD19 DART or anti-CD3/CD28 stimulation. It has been observed that granzyme B positive CD4+ produce IFNγ and have a Th1 phenotype.44 Whether CLL derived CD4+ T cells are indeed more skewed toward a Th2 phenotype remains to be elucidated, since conflicting results have been reported so far.45–47 Nevertheless, CD4+ T cells performed as well as CD8+ or a combination of CD4+ and CD8+ T cells in inducing lysis of JeKo-1 or allogeneic CLL samples. The fact that the CLL derived T cells were not HLA-matched might have contributed to the increased cytotoxic CD4+ T cell response that was observed. In an autologous setting, where HLA-mismatching is irrelevant, CD4+ T cells were equally potent in killing autologous CLL as CD8+ T cells. Interestingly, in this setting presence of both CD4+ and CD8+ in a 1:1 ratio significantly increased target cell lysis. Since it is mostly the CD8+ T cells that produce cytotoxic molecules like perforin and granzyme B, it shows that potent CD8+ mediated autologous antitumor responses can be further enhanced by the presence of CD4+ T cells, which can potentiate cytokine production.48 Nevertheless, it remains to be elucidated what would be the ideal T cell composition for optimal CD3xCD19 DART mediated responses. CAR T cell research has shown that 1:1 CD4:CD8 ratios in the CAR T cell product may be beneficial for clinical responses,49 50 although this does not seem to be the case for all CAR T cell products.51 Since CLL patients are reported to have a reduced CD4:CD8 ratio, this might be worthwhile to investigate.52 53
Cell death induced by both venetoclax and chemotherapy has been characterized as apoptosis. However, anti-apoptotic profiles in CLL are induced both in the LNs as well as on treatment with chemotherapy, due to upregulation of the anti-apoptotic molecules MCL-1 and BCL-XL or Bfl-1, respectively.29 54 Although venetoclax is effective, LN residing CLL cells are less sensitive to this drug, and mechanisms of resistance to this relatively new therapy have already been described.3 Our data indicate that after in vitro CD40L stimulation, a model mimicking the LN, venetoclax resistant CLL can still be targeted using CD3xCD19 DART. This indicates that CD3xCD19 DART mediated cell death is regulated differently compared with venetoclax or chemotherapy induced lysis, and therefore could be a valuable addition to the CLL therapy arsenal.
The observed CD3xCD19 DART mediated killing by T cells was granule dependent and therefore likely dependent on granzyme B. The present data demonstrate that CD3xCD19 DART mediated lysis is not dependent on BAX/BAK and therefore the intrinsic apoptotic pathway is not necessary to induce cell death, as shown previously for purified granzyme B and cytotoxic T cell mediated killing.55 56 Furthermore, cell death still occurred when caspases, and subsequent PARP cleavage, were blocked by using QVD-OPh, further indicating that CD3xCD19 DART mediated killing is non-apoptotic. Previously, similar T cell mediated cell death has been observed in an in vitro setting using mouse cytotoxic lymphocytes, in which caspase inhibition did not rescue cell death of peptide pulsed mouse embryonic fibroblasts.57
Where apoptosis is usually defined as a form of immunologically silent cell death, other forms like necrosis and necroptosis are generally proinflammatory.58 In immunotherapy settings, it therefore might be beneficial to induce a proinflammatory type of cell death to boost the immune system. Indeed, inducing intratumoral necroptosis of fibroblasts in a melanoma mouse model increased tumor-antigen loading on antigen presenting cells as well as durable tumor clearance in combination with ICB, indicating that inducing proinflammatory cell death leads to enhanced tumor clearance.59 However, it remains to be elucidated how cell death is characterized in CD3xCD19 DART-mediated setting on caspase inactivation, and whether this form of cell death is also relevant in an in vivo setting.
Conclusions
Our data indicate that CD3xCD19 DART therapy might be a feasible option for autologous based T cell therapy in CLL. CD3xCD19 DART exposure results in robust T cell stimulation and even venetoclax resistant samples are still sensitive to CD3xCD19 DART mediated lysis. Therefore, CD3xCD19 DART treatment might have potential in CLL in combination with targeted therapies or as salvation therapy after venetoclax relapse.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplementary information. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Ethics approval
The study was approved by the medical ethics committee at Amsterdam UMC (ethics approval number 2013/159).
Acknowledgments
The authors thank the patients and healthy donors for their blood donations.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
GJWvdW and APK contributed equally.
Correction notice Figure 5I has been updated. 1 patient was removed from the figure since the patient which was originally labeled as chronic lymphocytic leukemia, has been recently rediagnosed as indolent CD5+ mantle cell lymphoma.
Contributors AWJM, SRJ, HA, LI, SHT, EE, GJWvdW and AK designed research; AWJM, SRJ and IAMD performed research; AWJM and SRJ analyzed data; RvK provided patient samples and reviewed the paper; and AWJM, EE, GJWvdW and AK wrote the paper.
Funding This work was supported by the Netherlands Organisation for Scientific Research (NWO)/Netherlands Organisation for Health Research and Development (ZonMw) VIDI grant and Janssen Pharmaceuticals Companies of Johnson and Johnson.
Competing interests AK and EE have sponsored research grants from Janssen Pharmaceutical Companies of Johnson & Johnson. HA and LI are employees of Janssen Pharmaceutical Companies of Johnson & Johnson. GJWvdW is employee of Genmab.
Provenance and peer review Not commissioned; externally peer reviewed.