Article Text
Abstract
Background The extent to which response and survival benefits with immunotherapy-based regimens persist informs optimal first-line treatment options. We provide long-term follow-up in patients with advanced renal cell carcinoma (aRCC) receiving first-line nivolumab plus ipilimumab (NIVO+IPI) versus sunitinib (SUN) in the phase 3 CheckMate 214 trial. Survival, response, and safety outcomes with NIVO+IPI versus SUN were assessed after a minimum of 42 months of follow-up.
Methods Patients with aRCC were enrolled from October 16, 2014, through February 23, 2016. Patients stratified by International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk and region were randomized to nivolumab (3 mg/kg) plus ipilimumab (1 mg/kg) every 3 weeks for four doses, followed by nivolumab (3 mg/kg) every 2 weeks; or SUN (50 mg) once per day for 4 weeks (6-week cycle). Primary endpoints: overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) per independent radiology review committee in IMDC intermediate-risk/poor-risk patients. Secondary endpoints: OS, PFS, and ORR in the intention-to-treat (ITT) population and safety. Favorable-risk patient outcomes were exploratory.
Results Among ITT patients, 550 were randomized to NIVO+IPI (425 intermediate/poor risk; 125 favorable risk) and 546 to SUN (422 intermediate/poor risk; 124 favorable risk). Among intermediate-risk/poor-risk patients, OS (HR, 0.66; 95% CI, 0.55–0.80) and PFS (HR, 0.75; 95% CI, 0.62–0.90) benefits were observed, and ORR was higher (42.1% vs 26.3%) with NIVO+IPI versus SUN. In ITT patients, both OS benefits (HR, 0.72; 95% CI, 0.61–0.86) and higher ORR (39.1% vs 32.6%) were observed with NIVO+IPI versus SUN. In favorable-risk patients, HR for death was 1.19 (95% CI, 0.77–1.85) and ORR was 28.8% with NIVO+IPI versus 54.0% with SUN. Duration of response was longer (HR, 0.46–0.54), and more patients achieved complete response (10.1%–12.8% vs 1.4%–5.6%) with NIVO+IPI versus SUN regardless of risk group. The incidence of treatment-related adverse events was consistent with previous reports.
Conclusions NIVO+IPI led to improved efficacy outcomes versus SUN in both intermediate-risk/poor-risk and ITT patients that were maintained through 42 months’ minimum follow-up. A complete response rate >10% was achieved with NIVO+IPI regardless of risk category, with no new safety signals detected in either arm. These results support NIVO+IPI as a first-line treatment option with the potential for durable response.
Trial registration number NCT02231749.
- clinical trials, phase III as topic
- CTLA-4 antigen
- immunotherapy
- kidney neoplasms
- programmed cell death 1 receptor
Data availability statement
Data are available upon reasonable request. Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharingrequest-process.html. Deidentified and anonymized datasets of clinical trial information, including patient-level data, will be shared with external researchers for proposals that are complete, for which the scientific request is valid and the data are available, consistent with safeguarding patient privacy and informed consent. Upon execution of an agreement, the deidentified and anonymized data sets can be accessed via a secured portal that provides an environment for statistical programming with R. The trial protocol and statistical analysis plan will also be available. Data will be available for 2 years from the study completion or termination of the program (August 2024).
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- clinical trials, phase III as topic
- CTLA-4 antigen
- immunotherapy
- kidney neoplasms
- programmed cell death 1 receptor
Introduction
Recent approvals of immunotherapy-based regimens have revolutionized the treatment of patients with advanced renal cell carcinoma (aRCC).1 2 First-line aRCC treatments include the dual immune checkpoint inhibitor combination nivolumab plus ipilimumab (NIVO+IPI) as well as immunotherapy–tyrosine kinase inhibitor combinations, with numerous novel regimens under investigation.3–7 The extent to which response and survival benefits with immunotherapy-based regimens persist after long-term follow-up will inform optimal first-line treatment options.
NIVO+IPI combination therapy was the first to demonstrate superiority over sunitinib (SUN) in the first-line treatment of patients with International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate-risk/poor-risk aRCC.3 In the primary analysis of the phase 3 CheckMate 214 trial (minimum follow-up, 17.5 months), overall survival (OS) was superior (HR, 0.63; p<0.001) and confirmed objective response rate (ORR) was higher (42% vs 27%; p<0.001 per independent radiology review committee (IRRC)).3 Similar efficacy benefits were observed with NIVO+IPI in intention-to-treat (ITT) patients (any IMDC risk) and while ORR was higher with SUN in the exploratory favorable-risk population, OS outcomes were immature as of the primary analysis.3 After 30 months’ minimum follow-up, efficacy benefits with NIVO+IPI over SUN were maintained, including improved OS in both intermediate-risk/poor-risk and ITT patients, while the difference in OS outcomes between treatment arms was inconclusive in the favorable-risk subgroup.8
Here, we report additional follow-up through a minimum of 42 months to better inform the long-term impact of NIVO+IPI on clinical outcomes in the primary, secondary, and exploratory efficacy populations in CheckMate 214. Response per IRRC, durability of response, health-related quality of life, and characterization of safety were assessed with extended follow-up. Additionally, post hoc landmark OS analyses were conducted in patients with treatment-related adverse events (AEs) of interest and by response outcomes. This ongoing, multicenter trial enrolled patients between October 16, 2014, and February 23, 2016. These data represent the longest follow-up from a phase 3 trial of a dual immune checkpoint inhibitor regimen for aRCC reported to date.
Methods
Patients and treatment
CheckMate 214 is an ongoing, global, randomized, open-label, phase 3 trial; detailed methodology has been described previously.3 8 In brief, aRCC patients with a clear cell component were recruited from 175 hospitals and cancer centers in 28 countries, randomized 1:1 to the NIVO+IPI or SUN arm, and stratified by region and IMDC risk status (favorable, intermediate, or poor). Nivolumab 3 mg/kg and ipilimumab 1 mg/kg were administered intravenously every 3 weeks for four doses (induction), followed by nivolumab 3 mg/kg every 2 weeks (maintenance). SUN 50 mg was administered orally once per day for 4 weeks on and 2 weeks off in each 6-week cycle. Treatment continued until disease progression or unacceptable toxicity. A maximum of two dose reductions were permitted for SUN in 12.5 mg increments per day (daily dose must have been ≥25 mg); no dose reductions were allowed in the NIVO+IPI arm. Patients in the NIVO+IPI arm who developed a treatment-related AE that required discontinuation during the induction phase were taken off protocol and did not go on to receive maintenance nivolumab. The trial was stopped when NIVO+IPI demonstrated OS superiority over SUN in the primary efficacy population (August 7, 2017). A subsequent protocol amendment on November 13, 2017, permitted the following modifications in the NIVO+IPI arm: patients could discontinue after 2 years of study treatment even in the absence of disease progression or unacceptable toxicity; patients receiving nivolumab maintenance were permitted to switch to a flat dose of nivolumab (240 mg every 2 weeks); additionally, intermediate-risk/poor-risk patients could cross over to NIVO+IPI from SUN (online supplementary file 2).
Supplemental material
Assessments
The primary endpoints were OS, progression-free survival (PFS) per IRRC, and ORR per IRRC in intermediate-risk/poor-risk patients. Secondary endpoints were OS, PFS, and ORR in the ITT population and the incidence of AEs (per National Cancer Institute Common Terminology Criteria for Adverse Events, V.4.0) in all treated patients. Exploratory endpoints included efficacy in favorable-risk patients.3 This prespecified analysis reports updated OS, PFS, and ORR with duration of response in intermediate-risk/poor-risk (primary efficacy), ITT (secondary efficacy), and favorable-risk (exploratory) patients together with safety in all treated patients after extended follow-up. Response outcomes were confirmed and reported per IRRC using Response Evaluation Criteria in Solid Tumors (RECIST) V.1.1; best overall response was also assessed per investigator. Post hoc temporal analyses of treatment-related AEs, select treatment-related AEs, and corticosteroid use were conducted. Treatment-related select AEs were prespecified and defined as events that might be immune-mediated, differ from those caused by non-immunotherapeutic drugs, might require immunosuppression for management, and whose early recognition might mitigate severe toxicity (comprising events occurring in skin, gastrointestinal, endocrine, hepatic, pulmonary, or renal systems). Post hoc analyses were conducted to assess OS outcomes in ITT patient subgroups categorized by 6-month landmark events including response per RECIST V.1.1, any-grade immune-related AEs, and any-grade treatment-related AEs leading to discontinuation that occurred within 30 days of last dose. Immune-related AEs were defined as specific events regardless of causality that occurred within 30 days of last dose, required immune-modulating medication (or occurred in the endocrine system), and were considered immune-mediated by investigator assessment. Additional post hoc analyses included depth of response (≥50% reduction from baseline in sum of diameter of target lesions) in evaluable ITT patients at 6 months and detailed characterization of all complete responders (durability of response, treatment-free interval, and subsequent therapy outcomes). Treatment-free interval was defined as the time between protocol therapy discontinuation until subsequent therapy initiation or last known date alive. Health-related quality of life was assessed using National Comprehensive Cancer Network Functional Assessment of Cancer Therapy–Kidney Symptom Index (FKSI-19) scores.
Statistical analysis
After the planned interim analysis met the prespecified boundary of statistical significance for OS, it was considered the final primary analysis per protocol.3 Descriptive p values were included in the present analyses to confirm consistency with the primary analysis as appropriate. Here, OS, PFS, duration of study therapy, time to response, duration of response, and OS by 6-month landmark event were estimated using Kaplan–Meier methods.9 Stratified Cox proportional HRs and 95% CIs were calculated between treatment arms for OS and PFS (NIVO+IPI over SUN). ORR and the exact two-sided 95% CI were computed by Clopper–Pearson method; the two-sided p values were calculated per DerSimonian and Laird.10 11 Treatment-related AEs of interest were calculated overall and by 6-month interval using the total number of new events out of the total number of patients at risk at the beginning of the interval. The incidence of corticosteroid use (≥40 mg prednisone daily or equivalent (PDE)) for treatment-related select AE management over time was analyzed retrospectively using density plots summing vectors over time for patients in the NIVO+IPI arm. Each vector represents an individual patient’s time on treatment with corticosteroids. If a patient stopped and restarted corticosteroid treatment, the earliest and the latest dates of administration were used. Quality of life was assessed as an exploratory endpoint using patient-reported outcomes, including evaluation of disease-related symptoms based on the FKSI-19 scale. FKSI-19 scores range from 0 to 76; higher scores indicate fewer symptoms.12 13 For a given baseline score, quality of life was considered to have deteriorated if this score decreased by at least 1 threshold unit versus baseline. Time to confirmed deterioration was calculated using the Kaplan–Meier method. The univariate Cox model was used to calculate the HR with 95% CI. All statistical analyses were done with SAS V.8.2 or East V.5.4. This study is registered with ClinicalTrials.gov.
Results
A total of 1096 patients were randomized to NIVO+IPI (425 with intermediate-risk/poor-risk and 125 with favorable-risk disease) or SUN (422 with intermediate-risk/poor-risk and 124 with favorable-risk disease). Efficacy analyses were conducted in intermediate-risk/poor-risk, ITT (any risk), and favorable-risk patients. Overall, 547 patients in the NIVO+IPI arm and 535 in the SUN arm received treatment and were included in the safety analyses. Patients were enrolled from October 16, 2014, through February 23, 2016. The database lock for this analysis was August 7, 2019. At a minimum study follow-up of 42 months, 60 (11%) of 547 patients in the NIVO+IPI arm and 27 (5%) of 535 patients in the SUN arm continued therapy (online supplementary figure S1). Median follow-up for OS was 43.6 months in the NIVO+IPI arm and 32.3 months in the SUN arm (median of 39.3 months for the total study population).
Supplemental material
Key baseline characteristics were similar between treatment arms and across intermediate-risk/poor-risk, ITT, and favorable-risk patients, as reported previously (online supplementary table S1).3 8 Median duration of therapy (IQR) was 7.9 months (2.1–21.8) in the NIVO+IPI arm and 7.8 months (3.5–19.6) in the SUN arm. Treated patients in the NIVO+IPI arm received a median (range) of 14.0 doses (1–114) of nivolumab and 4.0 doses (1–4) of ipilimumab. Among all randomized patients, 51.8% (285/550) in the NIVO+IPI arm and 64.1% (350/546) in the SUN arm received subsequent systemic therapy. In the NIVO+IPI arm, subsequent systemic therapy included SUN (22.7%; 125/550), pazopanib (18.9%; 104/550), and axitinib (17.5%; 96/550). In the SUN arm, subsequent systemic therapy included nivolumab (38.6%; 211/546), axitinib (23.6%; 129/546), and cabozantinib (15.0%; 82/546).
In the primary efficacy population of intermediate-risk/poor-risk patients, OS was superior with NIVO+IPI versus SUN after extended follow-up (HR, 0.66; 95% CI, 0.55–0.80). The 42-month OS probability was 52% with NIVO+IPI versus 39% with SUN (figure 1A). An improvement in PFS benefit with NIVO+IPI versus SUN was observed (HR, 0.75; 95% CI, 0.62–0.90), and 42-month PFS probabilities were 33% versus 16%, respectively (figure 2A). ORR (95% CI) was 42.1% (37.4%–47.0%) with NIVO+IPI versus 26.3% (22.2%–30.8%) with SUN (table 1). In addition, a higher proportion of patients achieved a complete response (CR) with NIVO+IPI versus SUN (10.1% vs 1.4%). Similar results were observed per investigator assessment for ORR (42.4% vs 29.4%) and CR (12.2% vs 1.4%) (online supplementary table S2). Time to response was shorter and duration of response was longer with NIVO+IPI versus SUN (table 1; figure 2B). In the NIVO+IPI arm, 36/43 (83.7%) responses were ongoing in patients with CR and 85/136 (62.5%) responses were ongoing in patients with partial response (PR; table 1).
Overall survival. (A) In intermediate-risk/poor-risk patients. (B) In intent-to-treat patients. (C) In favorable-risk patients. mOS, median overall survival; NE, not estimable; NIVO+IPI, nivolumab plus ipilimumab; NR, not reached; SUN, sunitinib.
Progression-free survival and duration of response per independent radiology review committee. (A, B) In intermediate-risk/poor-risk patients. (C, D) In intent-to-treat patients. (E, F) In favorable-risk patients. mDOR, median duration of response; mPFS, median progression-free survival; NE, not estimable; NIVO+IPI, nivolumab plus ipilimumab; NR, not reached; SUN, sunitinib.
Confirmed objective response per independent radiology review committee in intermediate-risk/poor-risk patients, the ITT population, and in favorable-risk patients
Among ITT patients (secondary efficacy population), OS was also superior with NIVO+IPI versus SUN (HR, 0.72; 95% CI, 0.61–0.86), with 42-month OS probabilities of 56% versus 47%, respectively (figure 1B). A separation in the PFS curves was evident after ~24 months (HR, 0.88; 95% CI, 0.75–1.04), and 42-month PFS probabilities were 32% versus 20%, respectively (figure 2C). ORR (95% CI) was 39.1% (35.0%–43.3%) with NIVO+IPI versus 32.6% (28.7%–36.7%) with SUN, and a higher proportion of patients achieved CR with NIVO+IPI (10.7% vs 2.4%) (table 1). Investigator-assessed response was concordant in ITT patients (online supplementary table S2). Time to response was shorter and duration of response was longer with NIVO+IPI versus SUN (table 1; figure 2D). In the NIVO+IPI arm, 51/59 (86.4%) and 95/156 (60.9%) responses were ongoing in patients with CR and PR, respectively; in the SUN arm, 12/13 (92.3%) and 82/165 (49.7%) responses were ongoing in patients with CR and PR, respectively (table 1).
Among the exploratory efficacy population of favorable-risk patients, median OS was not reached in either arm and the HR for death was 1.19 (95% CI, 0.77–1.85); 42-month OS probabilities were comparable (70% with NIVO+IPI vs 73% with SUN; figure 1C). PFS benefits were observed with SUN (HR, 1.65; 95% CI, 1.16–2.35); the 42-month PFS probabilities were 27% with NIVO+IPI versus 33% with SUN (figure 2E). At the time of database lock, 48 of 125 versus 67 of 124 favorable-risk patients assessed for efficacy were progression free with NIVO+IPI versus SUN; 8 of 124 versus 11 of 119 treated patients remained on therapy in each arm, respectively. ORR (95% CI) was 28.8% (21.1–37.6) with NIVO+IPI versus 54.0% (44.9–63.0) with SUN, however, a higher proportion of patients achieved CR with NIVO+IPI versus SUN (12.8% vs 5.6%), and median (95% CI) duration of response was not reached (40.1–not estimable) versus 27.4 months (23.5–40.3), respectively (table 1; figure 2F). Responses were ongoing in 15/16 (93.8%) patients with CR and 10/20 (50.0%) patients with PR among favorable-risk patients in the NIVO+IPI arm (table 1). Some degree of discordance between response per IRRC and per investigator was observed, with ORR per investigator comparatively higher versus IRRC assessment in favorable-risk patients with NIVO+IPI (online supplementary table S2).
To better characterize long-term outcomes in complete responders, treatment-free interval and subsequent therapy were assessed among intermediate-risk/poor-risk and favorable-risk patients in both arms (figure 3). Among all 59 complete responders in the NIVO+IPI arm, 20 (33.9%) were still on therapy, 28 (47.5%) discontinued therapy with no subsequent systemic therapy, and 11 (18.6%) discontinued and then received subsequent systemic therapy. Among all complete responders, median (range) time to response was 2.8 months (0.9–9.8) and median duration of response was not reached with NIVO+IPI. Median (range) duration of study therapy was 47.5 months (40.5–53.2) among the 20 complete responders who remained on protocol therapy. Median (range) treatment-free interval was 34.6 months (0.5–49.7) among 28 complete responders who discontinued without subsequent systemic therapy.
Treatment-free interval and response outcomes in complete responders. (A) In intermediate-risk/poor-risk (top) and favorable-risk (bottom) patients in the NIVO+IPI arm. (B) In intermediate-risk/poor-risk (top) and favorable-risk (bottom) patients in the SUN arm. TFI was defined as the time between protocol therapy discontinuation until subsequent therapy initiation or last known date alive. Bar indicates time on treatment/TFI. Time zero corresponds to first treatment date. Of all-risk patients, 11 versus 6 received subsequent systemic therapy with NIVO+IPI versus SUN. These patients may have stopped therapy due to investigator-assessed progression or other protocol-specified reason such as toxicity (data not shown). The decision to start subsequent systemic therapy in either arm was made by the investigator based on expert opinion and treatment guidelines, and these data were not formally collected. ITT, intention-to-treat; NIVO+IPI, nivolumab plus ipilimumab; TFI, treatment-free interval in patients who are off study treatment.
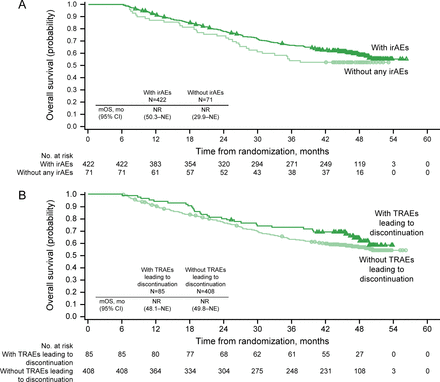
Exploratory post hoc analyses were conducted to assess long-term OS outcomes in ITT patient subgroups categorized by early response and treatment-related AEs at a landmark of 6 months. More evaluable ITT patients achieved a depth of response ≥50% maximal tumor reduction at 6 months with NIVO+IPI versus SUN (156/493 (31.6%) vs 65/472 (13.8%)). Similarly, more patients achieved a greater RECIST-defined response (CR or PR) with NIVO+IPI versus SUN at 6 months (198/481 (41.2%) vs 134/449 (29.8%) patients, respectively). A positive association was seen between RECIST-defined response at 6 months and OS in both treatment arms (figure 4). In the NIVO+IPI arm, OS probabilities at 42 months from randomization were 97% for patients with CR, 75% for patients with PR, 61% for patients with stable disease, and 27% for patients with progressive disease (figure 4A). Additionally, the association of AEs at 6 months with long-term survival was assessed in the NIVO+IPI arm among 493 ITT patients at risk. OS outcomes were similar between patients with immune-related AEs versus those without and were similar between patients who discontinued therapy due to any-grade treatment-related AEs versus those who did not, indicating that these AEs did not negatively affect long-term OS (figure 5).
Six-month landmark analysis of overall survival by best overall response per RECIST V.1.1 (per IRRC). (A) In the NIVO+IPI arm. (B) In the SUN arm. CR, complete response; IRRC, independent radiology review committee; NIVO+IPI, nivolumab plus ipilimumab; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; SUN, sunitinib.
Six-month landmark overall survival analyses in intent-to-treat patients with nivolumab plus ipilimumab. (A) Immune-related adverse events (irAEs), yes versus no. (B) Treatment-related adverse events (TRAEs) leading to discontinuation, yes versus no. Includes events reported between first dose and 30 days after last dose of study therapy. mOS, median overall survival; NE, not estimable; NR, not reached.
Consistent with previous reports,3 8 similar overall rates of treatment-related AEs of any grade occurred in the NIVO+IPI and SUN arms with extended follow-up (514/547 (94.0%) vs 521/535 (97.4%) patients). Yet, there were fewer grade 3–4 treatment-related AEs with NIVO+IPI versus SUN (47.3% vs 64.1%; online supplementary table S3). Treatment-related AEs leading to discontinuation within 30 days of last dose occurred in 121 (22.1%) patients in the NIVO+IPI arm and in 69 (12.9%) patients in the SUN arm. No additional treatment-related deaths were reported since the primary analysis: 8 (1.5%) in the NIVO+IPI arm and 4 (0.7%) in the SUN arm. The incidence of any-grade and grade 3–4 treatment-related AEs by 6-month interval was consistently lower with NIVO+IPI versus SUN over time (figure 6A). The overall incidence of treatment-related select AEs with NIVO+IPI was similar to previous reports3 8 (online supplementary table S4). The temporal patterns of treatment-related select AE incidence and corticosteroid use (≥40 mg PDE) both peaked within the first 6 months of treatment with NIVO+IPI (figure 6B,C). In total, 157 (28.7%) of 547 patients treated with NIVO+IPI received corticosteroids (≥40 mg PDE) to manage any-grade treatment-related select AEs; 104 (19.0%) patients received ≥40 mg PDE continuously for ≥2 weeks and 54 (9.9%) received ≥40 mg PDE continuously for ≥30 days.
Safety outcomes over time. (A) Treatment-related adverse events (AEs) over time by 6-month interval with nivolumab plus ipilimumab (NIVO+IPI) versus sunitinib (SUN). (B) Select treatment-related AEs over time by 6-month interval with NIVO+IPI. (C) Corticosteroid use over time with NIVO+IPI. aN=patients at risk at the beginning of each 6-month interval, and patients may be counted more than once across intervals. bIncidence of grade 3–4 treatment-related AEs in all intervals with NIVO+IPI after 24 months was ≤2.4%. cN=patients at risk at the beginning of each 6-month interval, and patients may be counted more than once across intervals. dIncidence of grade 3–4 treatment-related select AEs in all intervals with NIVO+IPI after 12 months was ≤1.6%. e≥40 mg prednisone daily or equivalent. Treatment-related AEs and treatment-related select AEs were calculated by 6-month interval using the total number of new events out of the total number of patients at risk at the beginning of the interval.
Kidney cancer–specific health-related quality of life benefits were observed with NIVO+IPI over SUN after extended follow-up, as measured using FKSI-19 questionnaires. The questionnaire completion rate was adjusted for study attrition and exceeded 80% for those who remained on protocol therapy for the duration of the study. Analyses of time to confirmed deterioration per FKSI-19 total score among intermediate-risk/poor-risk and ITT patients showed that NIVO+IPI significantly reduced the risk of worsening quality of life compared with SUN (intermediate/poor risk, HR, 0.64 (95% CI, 0.54–0.77); ITT, HR 0.64 (95% CI, 0.55–0.74)). Similarly, the risk was significantly reduced with NIVO+IPI in disease-related symptoms, physical disease-related symptoms, treatment side effects, and functional well-being domain scores (online supplementary figure S2).
Discussion
These results demonstrate long-term survival benefit and durable responses with NIVO+IPI over SUN after extended follow-up of greater than 42 months. OS and ORR benefits were maintained with NIVO+IPI over SUN in intermediate-risk/poor-risk patients and in the ITT population comprising all patients, regardless of risk category. Additionally, a PFS plateau emerged after 36 months at ~33% with NIVO+IPI in both intermediate-risk/poor-risk and ITT patients, further supporting the unique durable response seen with this dual checkpoint inhibitor regimen. Among favorable-risk patients, ORR was higher and median PFS was longer with SUN; yet, the differences in OS outcomes between treatment arms were not conclusive. Additionally, the CR rate was higher in the NIVO+IPI arm, and a separation in the duration of response curves emerged after ~18 months in favor of NIVO+IPI.
More responses were durable with NIVO+IPI versus SUN across all IMDC risk categories. A greater proportion of all patients achieved a CR with NIVO+IPI, and most of these CRs were durable at the time of database lock. Notably, almost half of all complete responders experienced a treatment-free interval without initiating subsequent therapy in the NIVO+IPI arm. Additionally, a greater proportion of patients had a deep response (≥50% maximal tumor shrinkage), and RECIST-defined ORR was higher with NIVO+IPI versus SUN among evaluable ITT patients at 6 months. Favorable RECIST-defined response at 6 months was positively associated with long-term OS with NIVO+IPI.
Neither incidence of immune-related AEs nor discontinuation of therapy due to treatment-related AEs at 6 months negatively impacted long-term OS with NIVO+IPI. Interestingly, a positive trend was observed between these AEs and OS, suggesting that these early events may be indicative of immune activation and could potentially be prognostic of response and long-term survival with NIVO+IPI. Previous studies have shown that immune-related events occurring early in the course of immune checkpoint inhibitor treatment may correlate with clinical benefit in several malignancies.14–17
No new safety signals were observed, and the overall incidence of treatment-related events with longer follow-up was similar to previous rates.3 8 Looking at treatment-related AEs by 6-month interval, the overall incidence decreased over time in both arms, yet consistently higher rates of any-grade and grade 3–4 AEs occurred with SUN versus NIVO+IPI. The incidence of treatment-related select (potentially immune-mediated) AEs and corticosteroid use (≥40 mg PDE) was highest within the first 6 months of treatment with NIVO+IPI before decreasing over time, and overall rates of both were similar to previous reports.8 Additionally, intermediate-risk/poor-risk and ITT patients (comprising all IMDC risk, including favorable-risk patients) reported health-related quality of life benefits with NIVO+IPI versus SUN, as measured by time to deterioration of FKSI-19 total and multiple domain scores.
CheckMate 214 was not designed to assess outcomes in each efficacy population with equal power nor was it designed to assess outcomes within treatment arms across individual-risk groups. The analyses of the IMDC favorable-risk subgroup were limited by the comparatively small number of patients and wide 95% CIs observed. Additionally, response outcomes in this analysis were aligned with the primary analysis and focused on IRRC assessment, which differed from the most recent interim analyses after 30 months’ minimum follow-up.8
Taken together, after an extended minimum follow-up of 42 months, OS benefits were maintained with NIVO+IPI versus SUN in both intermediate-risk/poor-risk and ITT patients, and OS results were not conclusive in the favorable-risk subgroup. Responses with NIVO+IPI were generally deep and durable regardless of IMDC risk-based prognosis. CR rates were higher, and a comparatively high proportion of ITT patients remained progression-free at 42 months with NIVO+IPI versus SUN. In summary, these results support NIVO+IPI as a first-line treatment option with the potential for durable response in patients with aRCC.
Data availability statement
Data are available upon reasonable request. Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharingrequest-process.html. Deidentified and anonymized datasets of clinical trial information, including patient-level data, will be shared with external researchers for proposals that are complete, for which the scientific request is valid and the data are available, consistent with safeguarding patient privacy and informed consent. Upon execution of an agreement, the deidentified and anonymized data sets can be accessed via a secured portal that provides an environment for statistical programming with R. The trial protocol and statistical analysis plan will also be available. Data will be available for 2 years from the study completion or termination of the program (August 2024).
Ethics statements
Ethics approval
CheckMate 214 was approved by the institutional review board or ethics committee at each site and conducted in accordance with Good Clinical Practice guidelines per the International Conference on Harmonisation. All patients provided written informed consent based on Declaration of Helsinki principles. The CheckMate 214 protocol is available in the Supplement (online supplementary file 2).
Acknowledgments
Medical writing support was provided by Jennifer Tyson, PhD, of Parexel and was funded by Bristol-Myers Squibb Company. We thank the patients who participated in this study, the clinical study teams, and representatives of the sponsor who were involved in data collection and analyses.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @DrChoueiri
Correction notice This paper has been updated since published to update Table S1 and Figure 6B.
Contributors RJM had full access to all data in the trialand takes responsibility for the integrity of the data and the accuracy of the data analysis. RJM and NMT contributed to the conception and design of the trial. All authors provided study materials or patients. MBM completed the statistical analyses. SSS reviewed the clinical data.
Funding This study was sponsored by Bristol-Myers Squibb Company and ONO Pharmaceutical Company Limited. Authors received no financial support or compensation for publication of this manuscript. The funders contributed to the study design, data analysis, and data interpretation in collaboration with the authors. The funders had no role in data collection. The funders provided financial support for editorial and writing assistance. Patients treated at the Memorial Sloan Kettering Cancer Center were supported in part by Memorial Sloan Kettering Cancer Center Support Grant (Core Grant, number P30 CA008748). The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health (grant P30 CA016672).
Competing interests The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. RJM reports consulting or advisory roles with Eisai, Exelixis, Genentech/Roche, Incyte, Lilly, Merck, Novartis, and Pfizer, and research funding/travel/accommodations/expenses from Bristol-Myers Squibb Company (BMS), Eisai, Exelixis, Genentech/Roche, Novartis, and Pfizer. BE reports honoraria, consulting, or advisory roles with BMS, EUSA Pharma, Ipsen, Novartis, Pfizer, and Roche. DFM reports consulting or advisory roles with Array BioPharma, BMS, Exelixis, Genentech/Roche, Lilly, Merck, Novartis, and Pfizer, and research finding from BMS (Inst), Genetech/Roche (Inst), Merck (Inst), Novartis (Inst), and Prometheus (Inst). OAF reports employment, stock or other ownership, and honoraria from Pfizer (I); consulting or advisory roles with BMS Chile and BMS Colombia; and travel, accommodations, expenses from Roche and Tecnofarma. BM reports honoraria from and advisory roles with Bayer, MSD, Merck Serono, Sanofi, Servie, AstraZeneca, Amgen, Janssen, Eisai, and Eli Lilly, and travel expenses from Merck Serono. TP reports honoraria from Merck, BMS, Pfizer, Roche, Ipsen, Novartis, Exelixis, and AstraZeneca, and research funding from AstraZeneca (Inst), Roche (Inst), and Novartis (Inst). FD reports research funding from Ipsen (Inst), Novartis (Inst), and Pfizer (Inst). ERP reports consulting or advisory roles with BMS, Genentech/Roche, Merck, Clovis, Exelixis, Seattle Genetics, Incyte, Janssen, and Flatiron Health; research funding from Astellas Pharma (Inst), AstraZeneca (Inst) BMS (Inst), Genentech/Roche (Inst), Merck (Inst), Peloton (Inst), and Pfizer (Inst); patents, royalties, and other intellectual property: US Patent Application No 14/588,503, pending, filed January 2, 2015; and the following other relationships: BMS (fees for development of educational presentations); Merck (fees for development of educational presentations); Roche (fees for development of educational presentations); Novartis (fees for development of educational presentations), and Exelixis (personal fees). PB reports consulting or advisory roles with BMS, Pfizer, MSD Oncology, Novartis, Ipsen, Roche, Janssen Cilag, and EUSA Pharma, and honoraria from Astellas Pharma and Amgen. HJH reports consulting or advisory roles with BMS, Pfizer, Exelixis, Merck, Corvus, Surface Oncology, Armo Biosciences, and Novartis, and research funding from BMS and Merck. SG reports consulting or advisory roles with Astellas, BMS, Novartis, Bayer, Pfizer, Exelixis, AstraZeneca, Janssen Oncology, Corvus Pharmaceuticals, Genentech/Roche, EMD Serono, and Sanofi, and research funding from Pfizer (Inst), Acceleron Pharma (Inst), Merck (Inst), Agensys (Inst), Novartis (Inst), BMS (Inst), Bayer (Inst), Eisai (Inst), and Corvus (Inst). VG reports consulting or advisory roles with BMS, Pfizer, Novartis, Lilly, MSD Oncology, Ipsen, Janssen Cilag, and Onkowissen; consulting or advisory roles with Bayer, BMS, Pfizer, Ipsen, and AstraZeneca; stock or other ownership in MSD, BMS, and AstraZeneca; honoraria from BMS, Pfizer, Novartis, Ipsen, Eisai, Bayer, MSD Oncology, Merck Serono, Roche, Lilly, PharmaMar, AstraZeneca, EUSA Pharma, Janssen Cilag, Asklepios Clinics, Diakonie Clinic, Dortmund Hospital, and Clinic of Oldenburg; and research funding from Novartis (Inst). CP reports consulting or advisory roles with BMS, MSD, Pfizer, Ipsen, EUSA, Eisai, and General Electric; speakers’ bureau fees from BMS, MSD, Pfizer, Ipsen EUSA, Eisai, General Electric, Janssen, and AstraZeneca; research funding from Pfizer (Inst); expert testimony fees from Pfizer and EUSA; and travel, accommodations, and expenses from Roche. VN reports honoraria from MSD and TEVA. AR reports consulting or advisory roles with Pfizer, BMS, Roche, Ipsen, Merck, and AstraZeneca; research funding from Pfizer (Inst); and non-financial support from Pfizer, BMS, Ipsen, Merck, MSD, and AstraZeneca. TKC reports research (Institutional and personal) funding from AstraZeneca, Alexion, Bayer, BMS/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc, Exelixis, Ipsen, Tracon, Genentech, Roche, Roche Products Limited, F. Hoffmann-La Roche, GlaxoSmithKline, Lilly, Merck, Novartis, Peloton, Pfizer, Prometheus Labs, Corvus, Calithera, Analysis Group, Sanofi/Aventis, and Takeda; honoraria from AstraZeneca, Alexion, Sanofi/Aventis, Bayer, BMS/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc, Exelixis, Genentech, Roche, Roche Products Limited, F. Hoffmann-La Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, UptoDate, Analysis Group, NCCN, Michael J Hennessy (MJH) Associates, Inc (Healthcare Communications Company with several brands such as OnClive, PeerView and PER), L-path, Kidney Cancer Journal, Clinical Care Options, Platform Q, Navinata Healthcare, Harborside Press, American Society of Medical Oncology, New England Journal of Medicine, Lancet Oncology, Heron Therapeutics, and Lilly; consulting or advisory roles with AstraZeneca, Alexion, Sanofi/Aventis, Bayer, BMS/ER Squibb and Sons LLC, Cerulean, Eisai, Foundation Medicine Inc, Exelixis, Genentech, Heron Therapeutics, Lilly, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, UptoDate, NCCN, and Analysis Group; and the following patents, royalties or other intellectual properties: International Patent Application No PCT/US2018/12209, entitled 'PBRM1 Biomarkers Predictive of Anti-Immune Checkpoint Response', filed January 3, 2018, claiming priority to US Provisional Patent Application No 62/445,094, filed January 11, 2017, International Patent Application No PCT/US2018/058430, entitled 'Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy', filed October 31, 2018, claiming priority to US Provisional Patent Application No 62/581,175, filed November 3, 2017. BIR reports research funding from BMS, Pfizer, Merck, GNE, Roche, Aveo, and AstraZeneca; consulting or advisory roles with BMS, Pfizer, GNE/Roche, Aveo, Novartis, Synthorx, Peloton, Compugen, Merck, Arravive, Surface Oncology, and 3D Medicines, and stock or other ownership in PTC Therapeutics. CKK reports honoraria from BMS, Pfizer, Ipsen, and Eisai; and consulting or advisory roles with BMS, Pfizer, Ipsen, Eisai, Roche, Astellas, and Janssen. SST reports consulting or advisory roles with BMS, Calithera Biosciences, and Prometheus Laboratories; and research funding from BMS (Inst), Calithera Biosciences (Inst), Merck (Inst), Nektar Therapeutics (Inst), Peloton Therapeutics (Inst), Jounce Therapeutics (Inst), Pfizer (Inst), Genentech (Inst), Prometheus Laboratories (Inst), ARGOS Therapeutics (Inst), and Clinigen (Inst). M-OG reports lecture or advisory roles with Novartis, BMS, Pfizer, Bayer, Astellas, Intuitive Surgical, Hexal, Apogepha, AstraZeneca, MSD Janssen Cilag, ONO Pharmaceuticals, Ipsen, Medac Merck, and Serono; and research funding from Novartis, BMS, and Intuitive Surgical. HG reports consulting or advisory roles with Astellas, BMS, Novartis, Pfizer, MSD, AstraZeneca, Ipsen, and Roche. RL-A reports personal fees from BMS, MSD, and Pfizer. PFG reports research funding from BMS, Pfizer, Merck, MSD, and Roche. AA reports research funding from BMS, Pfizer, Merck, and Exelixis, and speakers’ bureau fees from Pfizer, Exelixis, BMS, and Merck. YT reports honoraria from Pfizer, Novartis, ONO Pharmaceutical Co, Ltd, Astellas, BMS, and Chugai; consulting or advisory roles with ONO Pharmaceutical Co, Ltd, Novartis, and Taiho; and research funding from Takeda (Inst), Pfizer (Inst), ONO Pharmaceutical Co, Ltd (Inst), Astellas (Inst), Novartis (Inst), and Chugai (Inst). MBM reports employment with and stock or other ownership in BMS. SSS reports employment with and stock or other ownership in BMS. NMT reports research funding from BMS, Calithera Biosciences, Nektar Therapeutics, Exelixis, Pfizer, Novartis, Arrowhead Pharmaceuticals, Mirati Therapeutics, Takeda, Epizyme, and Eisai Medical Research; consulting, advisory, travel accommodations and expenses from BMS, Calithera Biosciences, Nektar Therapeutics, Exelixis, Pfizer, Novartis, Eisai Medical Research, Ipsen, Lilly Oncology, Neoleukin Therapeutics, Surface Oncology, ONO Pharmaceutical, and Oncorena; and honoraria from BMS, Exelixis, Nektar Therapeutics, Calithera Biosciences, Eisai Medical Research, ONO Pharmaceutical, Eli Lilly, Oncorena, Ipsen, and Surface Oncology.
Provenance and peer review Not commissioned; externally peer reviewed.