Article Text
Abstract
Background Immune-mediated diarrhea and colitis (IMDC) is currently diagnosed and monitored by evaluating clinical symptoms. Deep remission is determined by endoscopic and histologic evaluation of the disease process. However, repeating these invasive procedures frequently can become cumbersome. We sought to assess the role of fecal calprotectin (FC) concentration as a non-invasive biomarker of endoscopic or histologic remission.
Methods We performed a retrospective study of patients with IMDC who were tested for FC at IMDC onset and after IMDC treatment between June 2016 and March 2020. Patient demographics, clinical variables, and FC data were collected and analyzed to determine the optimal cut-off FC concentration to predict endoscopic and histologic remission.
Results Our sample comprised 77 patients with a median age of 62 years; 66% were male and 94% were Caucasian. Sixty-five patients (84%) achieved clinical remission, 46 (60%) achieved endoscopic remission, and 24 (31%) achieved histologic remission after IMDC treatment. FC concentrations decreased from the time of IMDC onset to the end of treatment (p<0.001). High FC concentrations were associated with evident endoscopic inflammation (p=0.003) and acute/chronic active colitis (p=0.025) which positively correlated with the Mayo Endoscopic Subscore (r=0.615, p=0.001) at the time of IMDC onset. In patients who achieved endoscopic remission after treatment, a significantly lower FC concentration was observed at IMDC onset (p=0.006) and after treatment (p<0.001) compared with those without endoscopic remission. The cut-off FC concentration to predict endoscopic remission was ≤116 μg/g and for histologic remission ≤80 μg/g; these cut-offs had optimal specificity (94% and 85%, respectively) and positive predictive value (0.91 and 0.38, respectively).
Conclusions FC concentration may serve as a non-invasive biomarker to predict endoscopic and histologic remission in patients receiving treatment for IMDC, minimizing the need for frequent invasive endoscopies. Future prospective studies are needed to provide further insight on the role of this marker in disease surveillance.
- immunotherapy
- inflammation
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Immune checkpoint inhibitors (ICIs), namely cytotoxic T cell lymphocyte-4 (CTLA-4) and programmed death-1/programmed death-1 ligand (PD-1/L1) inhibitors, bear substantial promise for patients with advanced malignancies, with established survival benefit.1 More than eight ICIs are approved by the US Food and Drug Administration as first-line therapy for advanced-stage malignancies.2 However, as the effects of immune checkpoint blockade on T cells are not specific to tumor antigens, any organ system in the body can be affected, leading to immune-related adverse events (irAE).3 Immune-mediated diarrhea and colitis (IMDC), a commonly encountered irAE, can lead to discontinuation of efficacious ICI therapy.4
Currently, oncologic guidelines for management of IMDC are based primarily on clinical symptoms.5 6 Common Terminology Criteria for Adverse Events (CTCAE) grade 2 irAE should prompt initiation of corticosteroid therapy. Grade 3 or higher irAEs indicate a need for hospitalization and corticosteroid-sparing therapy, namely infliximab and vedolizumab, until clinical remission has been achieved. Clinical symptoms are not always an accurate measure of disease severity.6–9 High-risk endoscopic features (presence of large, deep, and multiple ulcers and extensive inflammation) are associated with an increased risk of recurrence after clinical remission, frequent use of selective immunosuppressive therapy (SIT; ie, infliximab or vedolizumab), higher rates of hospitalization, and longer hospital stays.6 We demonstrated that fecal calprotectin (FC) as a stool inflammatory marker in patients with IMDC had a strong association with endoscopic severity.6 There is evidence10 showing that endoscopic and histologic remissions in IMDC are associated with a significantly lower recurrence rate. Frequent endoscopic evaluation in a population of immunocompromised patients with cancer, although warranted, is invasive and burdensome. Therefore, we investigated the role of FC as a non-invasive biomarker for measuring, monitoring, and surveying IMDC disease activity during treatment.
Calprotectin, a calcium-binding protein, accounts for 60% of cytoplasmic proteins in neutrophils.11 An elevated FC concentration is associated with an increased concentration of colonic mucosal neutrophils.12 Calprotectin concentration in feces is six times higher than in plasma, and FC may remain stable for up to 1 week at room temperature, a highly desirable quality for a biomarker of intestinal inflammation.13 Previous studies14 revealed that FC can predict endoscopic inflammation, although optimal cut-off values vary across studies. Growing evidence shows that FC can be used as a biomarker to assess endoscopic and histologic remission in inflammatory bowel disease (IBD).15 16 FC concentration also correlates with severity of endoscopic inflammation in patients with IMDC at the time of diagnosis.6 Whether FC can be used as a surrogate marker for endoscopic and histologic remission and guide IMDC treatment has not been studied.
To address this knowledge gap, we aimed to investigate the association between FC and endoscopic and/or histologic features of IMDC at the time of diagnosis and after treatment. We sought to define optimal cut-off concentrations to predict endoscopic and histologic remission after IMDC treatment to provide an alternative non-invasive biomarker to monitor disease status and minimize frequent endoscopic evaluations.
Methods
Patient cohort
Informed consent was waived. Patients with IMDC whose FC was evaluated at IMDC onset and after IMDC treatment (steroid therapy with adjunctive SIT) between June 2016 and March 2020 were included. Patients who did not undergo endoscopic evaluation at IMDC onset and repeat endoscopy after treatment or had a diagnosis of pre-existing IBD and mesenteric ischemia were excluded. All patients stopped ICI therapy after IMDC diagnosis. The detailed patient selection process is shown in online supplemental figure 1.
Supplemental material
Clinical data collection
All patient data were obtained from institutional electronic medical and pharmacy databases. Baseline demographic data and oncology variables were extracted. IMDC features including time of IMDC onset, clinical presentation, endoscopic and histologic findings, initial IMDC duration, and type and duration of treatment were also collected.
IMDC outcomes assessment
IMDC severity was graded according to CTCAE V.5.0.17 Clinical remission was defined as a sustained resolution of symptoms to grade ≤1. Endoscopic findings were described as mucosal ulceration, non-ulcerative inflammation (erythema, exudate, loss of vascular pattern, edema, friability, or erosions), or normal appearance. The Mayo Endoscopic Subscore was used to assess endoscopic activity at IMDC onset. Endoscopic remission was defined as a resolution of inflammation on repeat examination. Histologic patterns were categorized as acute active colitis, chronic active colitis, microscopic colitis, or normal. Histologic remission was described as a resolution of active inflammation, with or without chronic inactive inflammation, as determined by two experienced pathologists. IMDC recurrence was defined as recurrent symptoms of IMDC >1 month after resolution of initial IMDC episode.
FC measurement
Stool samples were collected by patients within 24 hours of the assay. FC concentration was tested as a standard laboratory protocol at IMDC onset and after treatment of IMDC using QUANTA Lite Calprotectin ELISA. FC ≤50.0 μg/g was considered negative, 50.1–120.0 μg/g borderline, and ≥120.1 μg/g as abnormal based on the criteria. The lower and the upper limits of detection for FC were 15.6 μg/g and 1000 μg/g, respectively. Consequently, all FC levels <15.6 μg/g and >1000 μg/g were considered as equal to 15.6 μg/g and 1000 μg/g, respectively. In patients who had multiple FC tests while receiving treatment for IMDC, the most recent value was recorded as the post-treatment FC concentration.
Statistical analysis
Statistical analyses were performed using SPSS V.24.0 software. Continuous variables were presented as mean and SD or median and IQR, and categorical variables were presented as frequencies and percentages. Differences between groups were compared using a paired or unpaired t-test. Correlation analysis was determined using the Spearman rank correlation coefficient (r). Overall predictive performance was estimated by the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. The Hanley-McNeil method was used to test the statistical significance of differences between AUC values. The Youden index (sensitivity+specificity–1) was calculated to determine the optimal cut-off FC concentration to predict endoscopic and histologic remission with relatively high specificity. Sensitivity and positive predictive value (PPV) were determined according to the diagnostic testing methodology. Risk factors for endoscopic inflammatory activity were assessed by logistic regression model with ORs and 95% CIs. All statistical tests were two-sided. P<0.05 was considered statistically significant.
Results
Patient characteristics
Among a total of 77 patients, 65 (84%) achieved clinical remission of IMDC, and 46 (60%) and 24 (31%) patients achieved endoscopic and histologic remission, respectively. Patients’ demographic and clinical characteristics are shown in table 1. The median age at IMDC diagnosis was 62 years. Of the patients, 51 (66%) were male and 72 (94%) were Caucasian. Genitourinary cancer was the most common malignancy, and most patients (83%) had stage IV disease. Concerning ICI therapy, 13 patients (17%) received CTLA-4 inhibitors, and 37 (48%) and 27 (35%) patients received PD-1/L1 inhibitors and combination therapy, respectively.
Baseline demographic and clinical characteristics of patients (N=77)
Patients’ IMDC-related characteristics are presented in table 2 and online supplemental tables 1 and 2. Fifty-two patients (68%) had grade 3–4 diarrhea and 37 (48%) had grade 3–4 colitis. Non-ulcerative inflammation was the most common endoscopic feature (38 patients, 49%). Chronic active colitis was the most common histologic feature (44 patients, 57%). The median Mayo Endoscopic Subscore was 2 (IQR 0–3). The median duration of steroid use was 38 days (IQR 29–47), with a median of 2 tapering attempts. SIT consisted of vedolizumab, infliximab and combination therapy in 46 (60%), 16 (21%), and 15 (19%) patients, respectively. Fifty-two patients (68%) received ≥3 doses of SIT.
Supplemental material
Association between clinical characteristics (categorical variables) and fecal calprotectin concentration at IMDC onset (N=77)
Association between clinical characteristics and FC concentration at IMDC onset
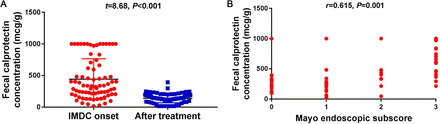
The mean (±SD) FC concentration decreased significantly after IMDC treatment (140±8 μg/g from 442±37 μg/g, p<0.001; figure 1A). A positive Spearman rank correlation was observed between the Mayo Endoscopic Subscore (at IMDC diagnosis) and FC concentration (r=0.615, p=0.001; figure 1B). Neither diarrhea nor colitis grade was associated with FC concentration at IMDC onset (table 2). The presence of endoscopic mucosal ulceration was associated with the highest FC concentration (641±71 μg/g), and significantly higher than the mean value in non-ulcerative inflammation (438±54 μg/g, p=0.031) and in normal-appearing colonic mucosa (263±44 μg/g, p<0.001). Acute and chronic active colitis were associated with higher FC concentration at IMDC onset compared with microscopic colitis (acute active colitis: 512±96 μg/g, chronic active colitis: 495±50 μg/g, microscopic colitis: 270±46 μg/g, p=0.025; table 2).
(A) Mean fecal calprotectin concentration at IMDC onset and after treatment of IMDC (paired t-test). (B) Spearman correlation between fecal calprotectin concentration and Mayo Endoscopic Subscore at IMDC diagnosis. IMDC, immune-mediated diarrhea and colitis.
Effect of treatment of IMDC on FC concentration
The median duration from first FC test at IMDC onset to last FC test after treatment was 9 months (IQR 8–13). The difference in FC concentration between IMDC onset and last test following treatment of IMDC was used to assess the effect of corticosteroid therapy and SIT. Neither duration (p=0.469) nor number of steroid therapy courses (p=0.623) was associated with a significant change in FC concentration. However, ≥3 doses of SIT were associated with a larger difference in FC concentration compared with 1–2 doses of SIT (p=0.033; online supplemental table 2).
Association between IMDC outcome and FC concentration
Sixty-five patients (84%) achieved clinical remission, and 46 (60%) and 24 (31%) patients achieved endoscopic and histologic remission of IMDC, respectively. Sixteen patients (21%) had recurrent IMDC. The groups with clinical and endoscopic remission at last follow-up had a significantly lower mean FC at IMDC onset compared with patients without remission (clinical remission: 406±38 μg/g compared with 641±98 μg/g, p=0.019; endoscopic remission: 361±44 μg/g compared with 563±59 μg/g, p=0.006; table 3). After treatment, a significantly lower mean FC was detected in patients with endoscopic and histologic remission than those who did not (endoscopic remission: 110±8 μg/g compared with 184±13 μg/g, p<0.001; histologic remission: 114±11 μg/g compared with 152±10 μg/g, p=0.029; table 3). This difference was not observed in patients with or without clinical remission (p=0.064) or IMDC recurrence (p=0.804; table 3). Univariate logistic regression analysis showed that endoscopic remission was associated with lower FC concentration (OR=0.97, 95% CI 0.96 to 0.99, p<0.001; online supplemental table 3). A lower mean FC was also observed in patients with clinical and histologic remission than in those without (p=0.051 and p=0.056, respectively; online supplemental table 3).
Association between IMDC outcome and fecal calprotectin concentration at IMDC onset and after treatment of IMDC (N=77)
ROC analysis and the predictive ability of FC
ROC analysis showed the AUC value for the ability of FC concentration after IMDC treatment to predict endoscopic and histologic remission was 0.828 (p<0.001) and 0.664 (p=0.022; figure 2A,B), respectively. ROC analysis with the Youden index criterion was performed to define the optimal cut-off FC concentration to predict endoscopic and histologic remission. For endoscopic remission, a cut-off of ≤116 μg/g had a specificity of 94% and a PPV of 0.91. For histologic remission, a cut-off of ≤80 μg/g had a specificity of 85% and a PPV of 0.38 (table 4).
(A–B) Receiver operating characteristic analysis showing AUC values in the ability of fecal calprotectin concentration to predict endoscopic (A) and histologic (B) remission (N=77). (C–D) Receiver operating characteristic analysis showing AUC values in the ability of fecal calprotectin concentration to predict endoscopic (C) and histologic (D) remission in the clinical remission group (n=65). AUC, area under the curve.
Performance of fecal calprotectin concentration in predicting endoscopic and histologic remission
Among patients who had clinical remission, a significantly lower mean FC concentration was observed in patients with endoscopic remission after treatment than in those without (110±8 μg/g compared with 187±19 μg/g, p<0.001), and the AUC values for the ability of FC concentration to predict endoscopic and histologic remission were 0.833 (p<0.001) and 0.685 (p=0.013), respectively (figure 2C,D). The optimal cut-off FC concentration for endoscopic remission among patients who achieved clinical remission was estimated as ≤140 μg/g, with a specificity of 89% and a PPV of 0.95, and the optimal cut-off for histologic remission among patients who achieved clinical remission was ≤80 μg/g, with a specificity of 80% and a PPV of 0.38 (table 4). Among 64 patients who had fecal lactoferrin (FL) tests (qualitative) at IMDC diagnosis and follow-up, the specificity of negative FL after treatment in predicting endoscopic remission was 54%, with a PPV of 0.48. The specificity of negative FL after treatment in predicting histologic remission was 52%, with a PPV of 0.15 (online supplemental table 4).
Discussion
irAEs present a major challenge in ICI therapy despite its exceptional outcomes in treating advanced malignancies. The evaluation and management of IMDC, as one of the most common irAEs, have been extensively studied.18 Endoscopic and histologic remissions after treatment are associated with low rates of recurrence.19 Frequent endoscopic assessment of IMDC activity, although invasive and cumbersome to a population of critically ill patients with cancer, provides optimal guidance for treatment decisions that yield improvements in long-term outcomes. To the best of our knowledge, this is the first study to show that FC can serve as an alternative non-invasive biomarker to monitor disease activity, to minimize the burden of frequent endoscopy.
Calprotectin, a calcium-binding and zinc-binding heterodimer, belongs to the S100 family,20 and is present on the surface of polynuclear neutrophils and macrophages cells, facilitating their recruitment to sites of inflammation.21 The synthesis of calprotectin is increased during inflammatory processes22 given its antimicrobial and immune-regulatory role via its interaction with zinc-dependent metalloproteinase.23 Although calprotectin is found in both plasma and feces, its concentration in the latter is much higher.24 The main advantage of FC is the derivation from direct mucosal contact that allows the detection of intestinal inflammatory conditions more precisely than serum biomarkers.25 FC is becoming the most useful non-invasive tool for monitoring the mucosal inflammation and assessing treatment response in IBD.
In our study cohort, we observe that active endoscopic inflammation at the time of IMDC diagnosis is associated with a significantly higher mean FC concentration, particularly in the presence of mucosal ulceration. This is consistent with prior findings, where FC significantly correlated with the Mayo Endoscopic Subscore (r=0.304, p<0.001) in cohorts of patients with ulcerative colitis and Crohn’s disease (r=0.61, p<0.001).26 27 Similar findings have also been reported in other IBD studies.28–32 Furthermore, this solidifies our work, which demonstrated that the non-invasive FC testing correlated with IMDC endoscopic findings6 and may serve as an initial cost-effective biomarker of colonic inflammation.
A significant decrease in FC from the time of IMDC onset to after treatment, accompanied by concurrent endoscopic and histologic remission, was noted in this cohort. To the best of our knowledge, a cut-off FC concentration to predict IMDC endoscopic and histologic remission has not been previously reported, although similar conclusions have been drawn regarding IBD. For instance, Theede et al29 established that FC concentration correlates with endoscopic and histologic activity in ulcerative colitis and can be used as a surrogate marker to identify patients with mucosal healing. They reported a cut-off of 192 μg/g correlated with the Mayo Endoscopic Subscore, with a PPV of 0.71 and a negative predictive value of 0.90. Taghvaei et al33 observed a mean FC of 194 μg/g in patients with mucosal healing; however, no predictive statistics were made. Cannatelli et al34 found that FC could predict endoscopic remission in patients with IBD, with the cut-off being 233 μg/g, with 100% sensitivity and 79% specificity. Lobatón et al35 reported that FC concentration <280 μg/g can identify mucosal healing with 75.4% sensitivity and 89.1% specificity. Overall, the cut-off FC concentration for predicting endoscopic remission ranged from 100 μg/g to 300 μg/g with different reference range. Our ROC analysis suggests a cut-off FC concentration of 116 μg/g to predict endoscopic remission, with an AUC of 0.828, specificity of 94%, and PPV of 0.91.
Several studies36–38 confirmed that FC can detect histologic remission and with threshold being lower than that of endoscopic remission. Although a validated cut-off FC to predict histologic remission has not been established, our results confirm the role of FC as a predictive marker for histologic remission in patients with IMDC. In our study, the optimal cut-off FC concentration to predict histologic remission was ≤80 μg/g, with an AUC of 0.664% and specificity of 85%. Although lower cut-off values allow for higher specificity and PPV, they are harder to achieve in practice. The high specificity of FC for prediction of endoscopic and histologic remission highlights its utility in disease monitoring and clinical decision-making.
IMDC therapy (steroid therapy and SIT) significantly reduced FC concentration. While corticosteroids are used for induction in IBD, their utility as agents for maintenance of remission is limited given lack of data and unfavorable side effect profile. In contrast, SITs, namely infliximab and vedolizumab, are efficacious biologics used for induction and maintenance to achieve endoscopic and histologic remission, as demonstrated in patients with IBD.39 40
FL may have utility in predicting endoscopic and histologic inflammation in IMDC. A meta-analysis of seven studies showed that FL has high sensitivity and specificity in suggesting the presence of gut inflammation.41 FL has been established as a predictive tool for endoscopic and histologic inflammation in IMDC, with 70% and 90% sensitivity, respectively; however, specificity after therapy is not ideal in predicting endoscopic and histologic remission (54% and 52%, respectively).6
Our study has several limitations. The retrospective study design provides suboptimal details of patients’ medication regimens and laboratory tests. Second, patients included comprised only those who received SIT and steroids. Our institutional guidelines42 for management of IMDC with mild symptoms do not recommend routine FC testing and endoscopic evaluation. Hence, the lack of a control group without SIT limits our assessment of the use of FC concentration in predicting endoscopic remission in those receiving steroid therapy alone. The small sample size, highly selective inclusion criteria, variation in SIT dosing and duration of follow-up may underestimate certain confounding factors, limiting our ability to perform subgroup analyses with adequate power.
Conclusion
FC concentration could serve as a predictive non-invasive biomarker to assess colitis disease status in patients with IMDC after treatment and aid in management decisions. FC concentrations of ≤116 μg/g and ≤80 μg/g may be the optimal cut-off values with high specificity to predict endoscopic and histologic remission, respectively, which can reduce the requirement for repetitive invasive endoscopic evaluation. The role of FC concentration in long-term IMDC management needs validation in future prospective studies.
Acknowledgments
Medical editing of this paper was provided by Erica Goodoff, Senior Scientific Editor at the Research Medical Library at The University of Texas MD Anderson Cancer Center.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @GlitzaMDPhD, @McQuadeMDLAc
Correction notice This paper has been updated to revise author affiliations.
Contributors YW and AT were the senior authors of this study; they developed the concept, designed the study, interpreted the results, ensured that the accuracy and integrity of the data were preserved at all stages, agreed to be accountable for all aspects of the study, were in charge of the overall direction and planning of the study, and contributed to the writing of the manuscript, with input from all authors. FZ collected the data for the study, compiled the external data, assessed the conduct and interpretation of data analysis, and wrote the manuscript. XW conducted the biostatistical analysis. IG, JM, JW, HCZ, and JT critically revised the final version of the manuscript. All authors read and approved the final manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval Approval of this study was granted by the Institutional Review Board at The University of Texas MD Anderson Cancer Center.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.