Article Text
Abstract
Background Although intravesical BCG is the standard treatment of high-grade non-muscle invasive bladder cancer (NMIBC), response rates remain unsatisfactory. In preclinical models, rapamycin enhances BCG vaccine efficacy against tuberculosis and the killing capacity of γδ T cells, which are critical for BCG’s antitumor effects. Here, we monitored immunity, safety, and tolerability of rapamycin combined with BCG in patients with NMIBC.
Methods A randomized double-blind trial of oral rapamycin (0.5 or 2.0 mg daily) versus placebo for 1 month was conducted in patients with NMIBC concurrently receiving 3 weekly BCG instillations (NCT02753309). The primary outcome was induction of BCG-specific γδ T cells, measured as a percentage change from baseline. Post-BCG urinary cytokines and immune cells were examined as surrogates for local immune response in the bladder. Secondary outcomes measured were adverse events (AEs) and tolerability using validated patient-reported questionnaires.
Results Thirty-one patients were randomized (11 placebo, 8 rapamycin 2.0 mg, and 12 rapamycin 0.5 mg). AEs were similar across groups and most were grade 1–2. One (12.5%) patient randomized to 2.0 mg rapamycin was taken off treatment due to stomatitis. No significant differences in urinary symptoms, bowel function, or bother were observed between groups. The median (IQR) percentage change in BCG-specific γδ T cells from baseline per group was as follows: −26% (−51% to 24%) for placebo, 9.6% (−59% to 117%) for rapamycin 0.5 mg (versus placebo, p=0.18), and 78.8% (−31% to 115%) for rapamycin 2.0 mg (versus placebo, p=0.03). BCG-induced cytokines showed a progressive increase in IL-8 (p=0.02) and TNF-α (p=0.04) over time for patients on rapamycin 2.0 mg, whereas patients receiving placebo had no significant change in urinary cytokines. Compared with placebo, patients receiving 2.0 mg rapamycin had increased urinary γδ T cells at the first week of BCG (p=0.02).
Conclusions Four weeks of 0.5 and 2.0 mg oral rapamycin daily is safe and tolerable in combination with BCG for patients with NMIBC. Rapamycin enhances BCG-specific γδ T cell immunity and boosts urinary cytokines during BCG treatment. Further study is needed to determine long-term rapamycin safety, tolerability and effects on BCG efficacy.
- urinary bladder neoplasms
- immunity
- innate
- immunotherapy
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
BCG is one of the first FDA-approved cancer immunotherapy agents. BCG treatment of bladder cancer was initially described in 1976.1 Half a century later, BCG continues to be the standard of care and the most effective therapy for bladder cancer in two scenarios: (1) to eradicate carcinoma in situ (CIS) and (2) to prevent disease relapse after endoscopic removal of papillary Ta and T1 bladder tumors.2 3 Despite BCG efficacy in treating bladder cancer, up to 40% patients do not respond to therapy and there are no approved combination strategies that boost BCG efficacy.4
Several properties of the mammalian target of rapamycin(mTOR) inhibitor, rapamycin, support testing in combination with BCG in patients with non-muscle invasive bladder cancer (NMIBC). First, rapamycin enhances BCG antigen presentation by promoting autophagy, thereby improving the vaccine efficacy of BCG against tuberculosis in preclinical models.5 6 Induction of BCG immunity during BCG treatment of bladder cancer, determined by purified protein derivative (PPD) testing, is associated with an improved clinical response.7 Thus, boosting of BCG specific immunity by rapamycin could improve BCG’s antitumor efficacy. Lack of efficacy of BCG is likely multifactorial, but may be related to age-dependent decline in immune function. As mTOR inhibition improves antigen-specific immunity,8 9 rapamycin could facilitate CD4+ and CD8+ T cell responses critical for BCG efficacy,10 resulting in improved BCG efficacy in elderly persons,11 an important consideration for bladder cancer patients who have a median age of 70 at diagnosis.12 mTOR inhibition can also improve T cell memory.13 For example, rapamycin boosts the immune response against EL4 lymphoma when combined with the immune checkpoint inhibitor anti-CTLA-4 and a tumor vaccine.14
In addition to conventional αβ T cells, γδ T cells are critical to BCG’s vaccine15 and antitumor activity.16 Unlike CD4+ and CD8+ T cells, γδ T cells recognize antigens in a major histocompatibility complex-unrestricted manner and are an important component of innate immune responses.17 γδ T cells are required for effective BCG treatment of mouse bladder cancer16 and BCG enhances cytotoxicity of γδ T cells against human bladder cancer.18 Our group and others have shown that rapamycin increases the proliferative capacity and effector function of γδ T cells.19–21 Moreover, rapamycin boosts human γδ T cell-mediated killing of human squamous cell carcinoma in a mouse xenograft model.20 Thus, rapamycin modulation of γδ T cells could boost BCG antitumor immunity.
These observations prompted testing of rapamycin in combination with BCG to treat bladder cancer. To investigate whether rapamycin affects γδ T cells in humans as predicted and to examine the safety and tolerability of rapamycin during routine BCG treatment, we conducted a randomized double-blind placebo-controlled clinical trial of oral rapamycin (0.5 mg or 2.0 mg) plus intravesical BCG in individuals with NMIBC. We tested the specific hypothesis that rapamycin would boost BCG-specific γδ T cells, measured as a percentage change from baseline.
Materials and methods
Clinical trial design and schema
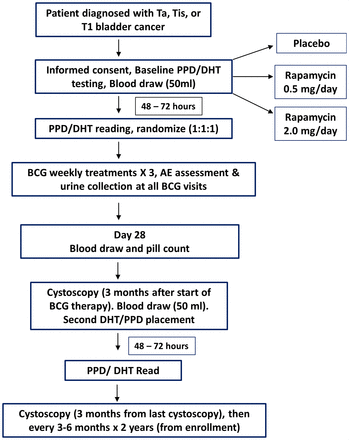
A double-blind, randomized, placebo-controlled trial was conducted and patient registration occurred between June 2016 and August 2018 (figure 1). Eligibility criteria included patients with NMIBC (that is, stage CIS, Ta, or T1) receiving intravesical maintenance TICE BCG. Patients were randomized to receive one of three agents using a 1:1:1 allocation ratio, including oral rapamycin (0.5 mg), oral rapamycin (2.0 mg), or placebo for 4 weeks starting immediately following a cystoscopy confirming no evidence of visible bladder tumors. During the month of study drug administration, patients also received intravesical maintenance BCG, including three weekly full-strength BCG treatments beginning approximately 1 week after starting study drug. The study was registered with clinicaltrials.gov (ID: NCT02753309) prior to activation.
Clinical trial schema. AE, adverse event; DHT, delayed hypersensitivity test.
A randomization list was generated using a computer-based pseudorandom number generator (https://www.sealedenvelope.com/simple-randomiser/v1/lists). Randomization was created with block size=11 and subjects were randomly assigned to receive placebo or one of two doses of oral rapamycin: 0.5 mg daily or 2.0 mg daily. Placebo and rapamycin tablets were reconstituted into a coated capsule by the pharmacist to conceal the identity of the agent. Rapamycin blood levels were quantified on day 30 of study, which was not a trough or peak draw but was considered random in relation to rapamycin dosing.
Safety and tolerability assessment
Safety. We assessed treatment safety using adverse event (AE) evaluation. AE data including onset, resolution, severity, attribution, and treatment (if necessary) were collected at each study visit. Study visits include baseline (day 1), intravesical BCG visits (days 7, 14, and 21), and follow-up (days 28 and 90). AEs were categorized and graded using the Common Terminology Criteria for Adverse Events V.5.0 and confirmed by the study principal investigator.
Tolerability. We measured tolerability based on changes in patient score of two validated bladder cancer questionnaires: (1) American Urologic Association Symptom Score (AUASS) administered weekly during intravesical BCG treatment, immediately prior to BCG and at day 28 from registration; (2) Bladder Cancer Index (BCI) quality of life (QoL) administered at baseline on the day of registration, days 28 and 90 following registration. Together, AUASS and BCI QoL scores measure function and bother (inconvenience) of urinary, bowel, and sexual health functions. BCI scores were calculated by standardizing Likert scale question responses to a 0 to 100 scale, where a score of 100 indicated the patient answered ‘no problem/bother’ to all questions; thus, higher scores represent better health states. AUASS and BCI scores were compared over time using a two-way analysis of variance with Tukey’s multiple comparison test.
Human biospecimen processing and storage
As described previously,18 blood was collected into heparin anticoagulated sterile tubes and centrifuged to remove plasma. Peripheral blood mononuclear cells (PBMCs) were isolated from the remaining whole blood by Ficoll-Paque (GE Healthcare) centrifugation and cryopreserved at −150°C until analyzed. Urine was collected 4–6 hours following BCG instillation weekly during induction and centrifuged to pellet down cells. Both urine cell pellets and aliquoted supernatants were stored immediately at −150°C and −80°C respectively until assayed.
BCG stock preparation
As described previously,18 TICE BCG organisms were cultured and prepared as follows: one vial of lyophilized BCG vaccine was reconstituted with 1 mL of sterile phosphate buffered saline. Approximately 50 µL of homogenous BCG suspension was then transferred into a T-25 flask with 5 mL of 7H9 broth (BD) containing 0.2% glycerol, 10% ADC Growth Supplement (5% bovine serum albumin, 2% dextrose and 0.9% NaCl; EMD Millipore) and 0.05% Tween-80 (Fisher Bioreagent) and cultured at 37°C with 5% CO2 for 7 days. When the BCG culture became turbid but with few visible clumps, the contents were transferred into a sterile 850 cm2 roller bottle (Celltreat Scientific Product) with up to 250 mL 7H9 broth with supplements. The BCG culture was kept on a roller at 30 rpm at 37°C with 5% CO2 for approximately 7 days when the culture started to form clumps and reached an OD600 absorbance of 0.8. BCG suspension was pelleted, washed with 7H9 broth, and aliquoted into 1 mL vials and stored at −80°C. After 48 hours, three vials of BCG were thawed, and each was diluted in 10-fold series to spread on a 7H10 agar plate (BD). Colony-forming unit (CFU) of frozen BCG stock were calculated based on the dilution that formed distinguishable individual colonies (20–100 CFU at 1–2 mm diameter) after culturing for 2 weeks at 37°C with 5% CO2.
Simultaneous detection of PBMC proliferation and IFNγ production in response to BCG
PBMCs (1×106) were labeled with carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes, Life Technologies) and expanded with an optimal dose of live TICE BCG (multiplicity of infection/MOI 0.1) in 1 mL of cR-10 containing 10% heat-inactivated human AB serum (MP Biomedicals), or resting in medium alone for 7 days at 37°C with 5% CO2 as described.18 On day 7, cell suspensions were mixed 1:500 with Cell Activation Cocktail (phorbol myristate acetate/PMA, ionomycin and Golgi blocker cocktail, BioLegend) for 5 hours. Cells were then processed for total live cell count (Vi-CellXR, Beckman Coulter) and staining with fluorochrome-conjugated anti-human CD3 mAb (clone: HIT3a, BioLegend), CD4 mAb (clone: OKT4, BioLegend), CD8 mAb (clone: RPA-T8, BioLegend) and γδ T cell receptor (TCR) mAb (clone: B1, BioLegend), followed by fixation and permeabilization with Cytofix/Cytoperm buffer (BD) prior to intracellular staining with fluorochrome-conjugated anti-human IFN-γ mAb (clone: 4S.B3, BioLegend). Data were acquired with an LSR II cytometer and analyzed using FACS Diva software (both BD). Absolute numbers (AN) of proliferated functional (defined as both CFSElo and IFN-γ+) CD4+, CD8+, and γδ T cells were calculated by multiplying total viable cells recovered after 7-day culture by percentages of proliferated functional T cell subsets.
Human urinary cytokine—luminex assay
Urine samples were diluted 1:20 with high-performance liquid chromatography grade water and measured for urinary creatinine level according to the manufacturer’s instructions using the Creatinine Colorimetric Assay Kit (Cayman Chemical) and a Synergy 2 plate reader (BioTek).18 Undiluted or diluted (1:1) samples were also run in duplicates using a Milliplex MAP 6-plex human cytokine panel (Millipore), and analyzed using FLEXMAP 3D and xPONENT software (Luminex). Average cytokine concentration of each urine sample was normalized based on its creatinine level to correct for bladder urine volume (at 100 mg/dL creatinine).
Human urine cells flow cytometry assay
Cryopreserved patient post-BCG urine cells were thawed and resuspended in cR-10 media at 10×106 cells/mL then passed through 100 µm strainer. Urine cell suspensions were either directly stained with flow cytometry antibody (for T cells and activation markers) or incubated with PMA/Ionomycin/Golgi Blocker (BioLegend, Cell Activation Cocktail) for 4 hours, followed by cell surface staining for T cells and then intracellular staining for cytokines. Flow staining antibodies/dye used were as follows: Fc blocker/Human TruStain FcX (BioLegend), fixable viability dye eFluor455UV (Thermo Fisher Scientific/eBioscience), anti-human CD45 (BioLegend, clone HI30), anti-human CD3, anti-human CD4, anti-human CD8, anti-human γδ TCR, anti-human TCR Vγ9 (BioLegend, clone B3), anti-human TCR Vδ2 (BioLegend, clone B6), anti-human CCR2 (BioLegend, clone KO36C2), anti-human CD44 (BioLegend, clone IM7), anti-human CD107a, anti-human CD56 (BioLegend, clone 5.1H11), anti-human IFNγ, anti-human TNFα, anti-human Granzyme B (BioLegend, clone GB11), anti-human Perforin (BioLegend, clone dG9). Fixed and stained urine cell samples were then passed through a 35 µm filter cap on a flow tube to remove any remaining debris before they were analyzed on an LSR II cytometer (BD).
Statistics
A three-arm randomized study of the immune effects of rapamycin in patients undergoing maintenance BCG therapy for bladder cancer was conducted. The primary study objective was to determine the effect of rapamycin on γδ T cell effector function in response to BCG. The sample size was calculated based on the primary immune endpoint, which was the number of BCG-specific γδ T cells (ie, γδ T cells that proliferated and produced IFN-γ in response to live BCG) in paired blood samples (baseline and post-treatment) among rapamycin-treated and placebo subjects. We tested the hypothesis that rapamycin-treated (either 0.5 mg or 2.0 mg daily for 4 weeks) patients would have a significantly increased mean percentage change in number of BCG-specific γδ T cells following treatment compared with patients that took the placebo. Our preliminary data, conducted in patients with bladder cancer receiving BCG without rapamycin, found a mean percentage increase in BCG-specific γδ T cells of 11.25% (SD 6.3%). Based on convincing preliminary laboratory findings and on published observations,11 we anticipated that rapamycin would result in an absolute increase of 8.5% in BCG-specific γδ T cells corresponding to total number change in BCG-specific γδ T cells of 19.5% (SD 6.3%). Our estimates rendered a necessary sample size of 30 completers, including 10 patients per treatment group, which achieves 81.4% power to detect a difference of 0.085 between placebo and rapamycin (at either dose) using a two-sided t-test at a significance level of 0.05. The SD in the placebo and treatment groups were assumed to be approximately equal. The study was blinded, and the random allocation was 1:1:1, and we allowed for one additional patient per group. Thus, the total enrolment per arm was targeted to be 11+11+11=33 with 30 completers. Sample size calculation was performed with Power Analysis and Sample Size (PASS) software.
Significance of urinary cytokine changes over time in each group of patients was assessed by linear regression analysis run on slope over weeks 1, 2, and 3. Differences in the change of BCG-specific responding T cell subsets in periphery at day 28 over baseline, or week 1 T cell subset percentages in the urine among patient groups were all assessed by t-test or Mann-Whitney test based on normality testing. P values are two-sided and p<0.05 was considered statistically significant. Statistical analyses were performed with GraphPad Prism 5–6 or Stata IC/10.1.
Results
Of the 33 patients enrolled, two subjects withdrew from the study prior to starting treatment. Therefore, a total of n=31 patients are included in the cohort analysis, including two patients who withdrew from protocol treatment secondary to AEs (table 1). The cohort included 27 (87%) men and 4 (13%) women. The majority of patients were non-Hispanic whites (n=23, 74%), while 8 (26%) Hispanics participated. Disease stage prior to treatment included pure CIS in 9 (29%), pure Ta in 19 (61%), pure T1 in 4 (13%), and mixed disease stage in 6 (19%) of the patients.
Baseline and pathologic characteristics of study patients
AEs and rapamycin blood levels are shown in online supplemental table 1. The median (IQR) rapamycin blood concentration for patients treated with 0.5 and 2.0 mg was 2.3 ng/mL (IQR, 1.7–5 ng/mL) and 3.05 ng/mL (1.8–10.7 ng/mL), respectively. Grade 1–2 AEs were observed in 21 patients (68%) and grade 3 AE occurred in 1 patient (4%), designated unrelated to rapamycin. No grade 4–5 AEs occurred. The number of AEs were similar between groups (table 2 and online supplemental table 2). The most common Grade 1 AEs were hematuria, dysuria, urinary frequency, and urgency (online supplemental table 1). Two patients (one receiving placebo and one receiving 2.0 mg rapamycin) experienced moderate (grade 2) mouth ulcers and/or sores, and were taken off treatment. Notably, AE severity was not associated with measured rapamycin blood concentration and no difference in moderate (grade 2) AEs was observed between groups (p=0.59). No difference was found in tolerability based on urinary function or bother during initial 4 weeks of the study (figure 2A–C) or bowel function or bother among all three groups (figure 2D,E).
Supplemental material
Supplemental material
Rapamycin is tolerated during intravesical BCG treatment. (A) AUA symptom scores tracked over the first 4 weeks of treatment. Mean±SEM. (B) BCI QoL questionnaire scores assessing urinary (B) function and (C) bother (inconvenience) and bowel habit (D) function and (E) bother (inconvenience). BCI scores were calculated by standardizing Likert scale question responses to a 0 to 100 scale, where a score of 100 indicated the patient answered ‘no problem/bother’ to all questions. Mean±SEM. AUA, American Urologic Association; BCI, Bladder Cancer Index; QoL, quality of life.
AEs were similar across treatment groups
The trial was not designed to assess treatment efficacy. Recurrence-free survival was similar between groups (p=0.35, (online supplemental figure 1). However, post-BCG induction of urinary cytokines, especially IL-2 and IL-8, is associated with a favorable response to BCG and is used as an early measure of treatment efficacy.22–25 No significant change in urinary IFN-γ occurred in any treatment groups (figure 3). Post-BCG urinary IL-8 levels progressively increased for all study groups, but this trend was statistically significant only for the rapamycin 2.0 mg group (figure 3). In this group, IL-8 increased from an average level of 1025 pg/mL (week 1) to 2659 pg/mL (week 2), and then 4205 pg/mL at week 3 of BCG therapy (p=0.02, figure 3). In addition, a significant increase in post-BCG urinary TNF-α was observed for patients receiving 2.0 mg rapamycin (figure 3). Interestingly, a progressive decrease in urinary IL-2 over time was observed for all groups and this trend was significant for patients receiving 0.5 mg rapamycin; this group had a substantially higher initial (week 1) urinary IL-2 compared with the other groups (figure 3).
Supplemental material
Rapamycin (2.0 mg daily) increases urinary IL-8 and TNF-α during intravesical BCG. Cytokines in post-BCG urine weeks 1–3 of BCG maintenance treatment detected by Luminex. P value, linear regression on slope over time for each group. Mean±SEM.
Urine immune cells reflect the constituency of bladder-infiltrating immune cells, thereby providing a surrogate and non-invasive measurement of bladder tumor environment effects and immune response to treatment.26 No significant difference in the percentage of urinary CD4+ or CD8+ T cells was observed between the treatment groups during the study (figure 4A). However, patients treated with rapamycin at either 0.5 or 2.0 mg daily experienced a significant increase in urinary γ9δ2 T cells compared with patients receiving placebo (figure 4A). Compared with other lymphocytes in the urine, γ9δ2 T cells are rare—the mean percentage is less than 0.01% even after BCG restimulation. However, these cells were significantly increased in patients treated with rapamycin. The average percentage of urinary γ9δ2 T cells were 4–5-fold higher than placebo (0.05% in 0.5 mg rapamycin group and 0.06% in 2.0 mg rapamycin group). Compared with patients receiving placebo, patients receiving 0.5 mg of rapamycin showed an approximate fivefold increase in the average percentage of activated CD44+ and CD107a+ degranulating (ie, functionally active) urinary γδ T cells (figure 4B). CD107a+ mean fluorescence intensity of degranulating γδ T cells, which is proportional to the number of exocyted granules per cell, was also significantly increased in the rapamycin 0.5 mg group compared with the placebo group (figure 4C,D). Because γδ T cells can regulate natural killer (NK) cell antitumor function,27 28 we examined effects of rapamycin on NK cells. While rapamycin did not appear to affect the number of urinary NK cells (figure 4A), the NK cell activation marker CD56 was significantly increased in its expression on urinary NK cells from patients treated with 2.0 mg of rapamycin in contrast to CD44 expression (figure 4E,F). These data indicate that rapamycin largely induces innate effector immunity in the bladder environment, including γδ T cell proliferation/activation and NK cell activation, without significant effects on bladder CD4+ or CD8+ T cells.
Increased urinary γδ T cells in response to BCG treatment in patients treated with rapamycin. (A) Percentage of each T cell subset and (B) activated or degranulating γδ T cells were analyzed among cells collected from week 1 post-BCG urine pellets by flow cytometry. Expression level of (C,D) CD107a and CD44 on γδ T cells and (E,F) CD44 and CD56 on NK cells from week 1 post-BCG urine pellets were also shown as MFI. γδ T cell, CD4+ and CD8+ T cell populations were gated under live CD45+ and CD3+ cells in urine, and NK cells were gated as CD45+CD3+CD56+ cells. P value indicates t-test or Mann-Whitney test, based on normality, for placebo versus rapamycin-treated group. Mean±SEM. MFI, mean fluorescence intensity.
Durable BCG-specific immunity requires T cells with long-term homeostatic proliferation capacity that can proliferate and produce effector cytokines in response to BCG antigen.29–33 The study’s primary endpoint was to assess rapamycin’s effect on the number of BCG-specific T cells in circulation and their capability for functional expansion, estimated using CFSE dilution to track lymphoproliferation combined with IFN-γ production in response to co-culturing the cells with live TICE BCG (figure 5A) as described.18 34 Compared with patients receiving placebo, the group treated with 2.0 mg rapamycin experienced a significant increase in the number of BCG-specific γδ T cells over baseline (p=0.03) (figure 5B). Patients receiving 0.5 mg daily rapamycin also experienced an increase in the number of BCG-specific γδ T cells compared with the placebo group, but this difference was not statistically significant (p=0.18) (figure 5B). The median (IQR) percentage change in BCG-specific γδ T cells from baseline per group was −26% (−51% to 24%) for placebo group, 9.6% (−59% to 117%) for rapamycin 0.5 mg group (versus placebo, p=0.18), and 78.8% (−31% to 115%) for rapamycin 2.0 mg group (versus placebo, p=0.03). No significant changes were observed in circulating BCG-specific CD4+ or CD8+ T cells in patients taking either dose of rapamycin (figure 5B). These data support rapamycin induction of BCG-specific γδ T cell immunity without significant effects on conventional T cells.
Increased proliferation and function of circulating γδ T cells by combination treatment of BCG and rapamycin. PBMCs from both baseline and day 28 were cocultured with BCG for 7 days. Proliferation and cytokines were measured by flow cytometry. Shown are (A) a representative example of visit 6 (day 28) response of one patient from each group (with highest response in γδ T cells) and (B) AN of proliferated IFNγ producing T cells were calculated based on day 7 live cell count multiplied by the percentage of the population. Then, changes of day 28 response over baseline were calculated and compared between Placebo group and each rapamycin group (bottom graph). P value indicates t-test or Mann-Whitney test, based on normality, for placebo versus each rapamycin-treated group. Mean±SEM. PBMC, peripheral blood mononuclear cells.
Discussion
In this study, rapamycin at a dose of 0.5 or 2.0 mg daily for 4 weeks, was tolerated when given in combination with intravesical BCG in patients with high-grade NMIBC. Rapamycin-related side effects were generally mild in severity and the AE profile was not unexpected based on contemporary clinical studies of intravesical BCG monotherapy.35 The study met its primary endpoint in demonstrating a greater increase in peripheral blood BCG-specific γδ T cells in subjects receiving 2.0 mg rapamycin compared with subjects receiving placebo. Further, the effects of rapamycin on circulating immune cells were analogous to changes observed in the local bladder environment, including γδ T cell proliferation and activation without significant effects on conventional T cell immunity. Importantly, rapamycin boosted BCG-specific immunity in patients during the maintenance phase of BCG treatment, indicating that rapamycin could help provide sustained BCG-specific immunity for patients with bladder cancer.
The safety and tolerability of rapamycin demonstrated in this patient population form the foundation for further study in this indication. At the low doses tested (ie, ≤2.0 mg daily), oral rapamycin was well-tolerated with no treatment-related grade ≥3 AEs noted. We specifically measured patient-reported urinary and bowel bother and function with genitourinary- and bladder cancer-specific validated questionnaires, which were not adversely affected in subjects receiving rapamycin compared with placebo. One subject receiving rapamycin discontinued treatment due to stomatitis, but this toxicity may be mitigated by strategies such as dexamethasone mouthwash.36 These data support the tolerability and feasibility of a short-course of rapamycin co-administered with BCG.
In addition to enhancing γδ T cell function, rapamycin could enhance bladder NK cell activity, either directly or via γδ T cell induction. γδ T cells regulate antitumor NK cell function,27 28 including against human bladder cancer.18 Both γδ T cells and NK cells are required for BCG’s antitumor efficacy.16 37 We reported that bladder-infiltrating NK cells expressing high levels of CD56 had increased functional capacity than their CD56dim counterparts.38 Although rapamycin had no effect on the number or percentage of urinary NK cells, rapamycin did increase the CD56 expression on NK cells, which supports a favorable NK cell phenotype in bladder tumors.
Given these data on the favorable immune modulation, safety, and tolerability of rapamycin coadministered with BCG, further studies of this combination are indicated in NMIBC. Future studies should assess the durability of immune cell modulation, including effects on γδ T cells and NK cells in the absence of ongoing rapamycin therapy. In murine models, low-dose intermittent rapamycin has been shown to increase lifespan and contribute to cancer prevention.39 40 Reducing the dose of rapamycin and restricting the dosing to a more intermittent regimen may improve its safety profile. For example, intermittent rapamycin dosing decreased its negative effects on glucose metabolism and the immune system relative to daily rapamycin treatment.41
Because rapamycin enhances BCG antigen peptide processing and presentation,5 initiating rapamycin earlier in the course of BCG therapy (ie, during BCG induction) could enhance BCG-specific immunity even further. Additionally, there may be a role for rapamycin coadministered with BCG in patients who have already experienced BCG failure, where the current standard is radical cystectomy. There are numerous strategies currently being investigated to salvage patients BCG unresponsive NMIBC. Rapamycin plus BCG may be studied as an alternative or in combination with such approaches.
We observed a high variability of rapamycin blood levels in treated patients. Further improvement in tolerability is possible with rapamycin dose adjustment based on measuring whole blood trough levels as is currently done for transplant patients or by using microencapsulated rapamycin which protects the agent from stomach digestion.42 While this trial was not designed to demonstrate the clinical efficacy of rapamycin combined with BCG, based on our prior work showing that the antitumor effect of BCG is mediated through γδ T cells, the urinary cytokine and immune cell changes observed with this combination are promising. Another limitation of the study was the absence of information on pretreatment urinary immune cells and cytokine levels, which are important measures as internal controls. Incorporation of pretreatment urinary data in future studies will aid the ability to correlate both baseline and BCG-induced immune parameters with clinical outcomes.
In summary, rapamycin had an acceptable safety profile in patients with high-grade NMIBC at doses 0.5 or 2.0 mg daily for 4 weeks. The immunomodulatory effects of rapamycin in blood and urine were encouraging and further plans for phase II testing of long-term eRapamycin plus intravesical BCG are underway (ClinicalTrials.gov Identifier: NCT04375813).
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @SvatekRob
Contributors NJ: design and perform immune studies, data acquisition and analysis, drafting and editing manuscript. NM: drafting/editing manuscript, assisting sample processing for immune analysis. RMR: clinical data analysis, drafting/editing manuscript. JG: data and statistical analysis, editing. MJ: editing. JM: editing. DM: editing. Z-JS: sample processing for immune data acquisition. CR: editing. RD: urine sample cytokine Luminex and creatinine data acquiring and analysis. TC: concept, design and editing. RS: key concept, design, drafting and editing.
Funding (1) 8KL2 TR000118, K23, (2) the Mays Family Cancer Center at University of Texas Health San Antonio (P30 CA054174), (3) Roger L. and Laura D. Zeller Charitable Foundation Chair in Urologic Cancer, (4) Max & Minnie Tomerlin Voelcker Fund, (5) CDMRP CA170270/P1P2, (6) Bladder Cancer Advocacy Network 2016 Young Investigator Award, (7) Research Training Award (RP170345) from Cancer Prevention & Research Institute of Texas, (8) MSTP Program (NIH T32GM113896), (9) NIH/NCATS TL1 TR002647, (10) NIA T32 AG 021890, (11) Glenda and Gary Woods Endowment in Genitourinary Oncology
Competing interests The studies related to this manuscript were conducted abided by research ethics and approved by IRB guidelines for clinical sample use. All authors have consent for publishing the data from the studies. RS discloses other roles as Consultant for FerGene and Clinical Research Support for JBL(SWOG), FKD and Decipher Biosciences. All other authors disclose no conflict of interest.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement All data relevant to the study are included in the article or uploaded as supplementary information.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.