Abstract
Aims/hypothesis
It is not known whether the beneficial effects of exercise training on insulin sensitivity are due to changes in hepatic and peripheral insulin sensitivity or whether the changes in insulin sensitivity can be explained by adaptive changes in fatty acid metabolism, changes in visceral fat or changes in liver and muscle triacylglycerol content. We investigated the effects of 6 weeks of supervised exercise in sedentary men on these variables.
Subjects and methods
We randomised 17 sedentary overweight male subjects (age 50 ± 2.6 years, BMI 27.6 ± 0.5 kg/m2) to a 6-week exercise programme (n = 10) or control group (n = 7). The insulin sensitivity of palmitic acid production rate (Ra), glycerol Ra, endogenous glucose Ra (EGP), glucose uptake and glucose metabolic clearance rate were measured at 0 and 6 weeks with a two-step hyperinsulinaemic–euglycaemic clamp [step 1, 0.3 (low dose); step 2, 1.5 (high dose) mU kg−1 min−1]. In the exercise group subjects were studied >72 h after the last training session. Liver and skeletal muscle triacylglycerol content was measured by magnetic resonance spectroscopy and visceral adipose tissue by cross-sectional computer tomography scanning.
Results
After 6 weeks, fasting glycerol, palmitic acid Ra (p = 0.003, p = 0.042) and NEFA concentration (p = 0.005) were decreased in the exercise group with no change in the control group. The effects of low-dose insulin on EGP and of high-dose insulin on glucose uptake and metabolic clearance rate were enhanced in the exercise group but not in the control group (p = 0.026; p = 0.007 and p = 0.04). There was no change in muscle triacylglycerol and liver fat in either group.
Conclusions/interpretation
Decreased availability of circulating NEFA may contribute to the observed improvement in the insulin sensitivity of EGP and glucose uptake following 6 weeks of moderate exercise.
Similar content being viewed by others
Introduction
Although it is well known that established obesity is associated with insulin resistance, this association has also been reported in non-obese, but overweight individuals (BMI 25–30 kg/m2) [1]. Specific factors which predispose these subjects to insulin resistance have been shown to be central fat distribution (both visceral and subcutaneous), also referred to as upper-body obesity, and low physical activity. Increased visceral fat may lead to the development of hepatic insulin resistance by increasing release of NEFA into the portal circulation [2], while increased subcutaneous fat may lead to release of more NEFA into the systemic circulation potentially leading to both peripheral and hepatic insulin resistance [3]. Fatty acid flux has been shown to be greater in obese individuals with upper-body obesity than in subjects with lower-body obesity [4, 5].
Elevated NEFA concentrations may cause insulin resistance by two mechanisms: substrate competition and inhibition of insulin signalling. Until recently it was considered that NEFA caused insulin resistance by the Randle cycle. This cycle proposed that increased NEFA oxidation restrained glucose oxidation in muscle by altering the redox potential of the cell and inhibiting key enzymes within the glycolytic cascade [6]. However, studies with 1H-magnetic resonance spectroscopy (1H-MRS) have shown that experimental elevation of NEFA concentration in plasma results in primary inhibition of glucose transport and phosphorylation in muscle [7, 8]. There is evidence that this is due to inhibition of insulin signalling via phosphatidylinositol 3-kinase [8]. Increased NEFA concentrations have also been shown to enhance gluconeogenesis thus increasing endogenous glucose production (EGP) [3, 9], and there is evidence that this may also be due to inhibition of insulin signalling [10]. Finally, increased NEFA have been shown to increase insulin secretion of the beta cell in the pancreas [11].
Increased circulating NEFA may lead to an increased deposition of triacylglycerol in muscle and liver and this may also lead to insulin resistance in these tissues. Insulin-stimulated glucose uptake is inversely correlated to intramyocellular lipid (IMCL) in normal subjects and offspring of type 2 diabetic patients [12] and subjects with increased liver fat have been shown to have reduced insulin sensitivity of EGP [13].
Exercise is a very effective way of improving insulin sensitivity. A 6-week exercise programme has been shown to increase insulin sensitivity by 40% in inactive subjects [14]. Although these authors did not separate the effect of exercise on insulin sensitivity into effects on EGP and peripheral glucose uptake the demonstration that exercise increased insulin-stimulated glycogen synthesis and insulin-stimulated glucose transport-phosphorylation in muscle suggests increased insulin sensitivity of peripheral glucose disposal [13]. The mechanism for the improved insulin sensitivity of glucose metabolism following exercise is not clear. Some studies suggest that it may be related to a decrease in circulating NEFA [15] and a reduction in central obesity [16]. Since recent evidence suggests that insulin resistance may be due to the accumulation of lipid in liver and muscle, exercise-induced changes in this may also be a possible mechanism.
We investigated the effect of a 6-week supervised exercise programme on the insulin sensitivity of palmitic acid and glycerol metabolism (a measure of lipolysis), EGP and peripheral glucose uptake, intrahepatocellular lipid (IHCL) and IMCL and visceral obesity in sedentary male subjects. To study the chronic rather than the acute effects of exercise training, studies were undertaken >72 h after the last exercise bout. Since EGP and lipolysis are sensitive to much lower concentrations of insulin than glucose uptake a two-step euglycaemic–hyperinsulinaemic clamp was used to measure insulin sensitivity. For the first step an insulin infusion rate of 0.3 mU kg−1 min−1 was used to measure the insulin sensitivity of EGP, glycerol and palmitic acid production rate (Ra); for the second step a high dose insulin infusion rate of 1.5 mU kg−1 min−1 was used to measure the insulin sensitivity of glucose uptake.
Subjects and methods
Subjects
Sedentary healthy male adult subjects (n = 17) with BMI 25 to 30 kg/m2 were recruited in response to an advertisement at King’s College London. Subjects were excluded if they had diabetes mellitus, were taking lipid-lowering medication or had clinically significant hyperlipidaemia (plasma triacylglycerol >3.95 mmol/l or total cholesterol levels >7.76 mmol/l). Subjects with significant cardiorespiratory or locomotor problems precluding vigorous exercise and subjects already engaged in regular physical activity were also excluded. The study was approved by St Thomas’ Hospital research ethics committee and all subjects gave informed consent.
The subjects were randomly allocated to an exercise group (n = 10) and a control group (n = 7). Subjects were interviewed by an exercise physiologist to establish that they were sedentary (defined as spending most of the day sitting and not participating in any recreational exercise).
Supervised training protocol
Subjects allocated to the exercise group exercised at 60 to 85% of maximal aerobic capacity (VO2max) for a minimum of 20 min at least three times per week for 6 weeks, as detailed in the Electronic supplementary material (ESM 1). The control group was asked to continue with their normal activity for the duration of the 6-week period. Both groups were asked not to change their dietary habits during the study.
VO 2max VO2max was measured using a step-wise maximal exercise test on an electronically braked bicycle ergonometer [17] as detailed in the ESM 1.
Study protocol
Subjects were studied on two occasions, before (0 week) and after a 6-week exercise programme or control period. Subjects were asked not to undertake any vigorous physical activity for 72 h [18] and to fast overnight before the study day. On the day of the study, the subject was weighed and intravenous cannulae were inserted into the antecubital fossa of each arm: one for blood-taking and the other for the infusion of the isotopes, insulin and exogenous glucose. After taking initial (unenriched) blood samples, an infusion of [1-13C]palmitate bound to human albumin (5%) (Grifols, Barcelona, Spain) [19, 20] (0.5 mg kg−1 h−1), and primed infusions of [1,1,2,3,3-2H5]glycerol (0.12 mg/kg; 0.4 mg kg−1 h−1) and [6,6-2H2]glucose (170 mg; 1.7 mg/min) were started. Once a steady state with the stable isotopes was achieved, a further five baseline samples were taken between 100 and 120 min to measure the enrichment of palmitic acid, glycerol and glucose, and concentrations of insulin, glucose, palmitic acid, NEFA and glycerol [20]. Samples were also taken for the measurement of adiponectin and glucagon concentration at baseline (100–120 min).
Three subjects from the exercise group and one from the control group did not receive [1-13C]palmitate due to problems with preparing this for the study.
At 120 min a two-step hyperinsulinaemic–euglycaemic clamp was started. Step 1 consisted of insulin infusion at 0.3 mU kg−1 min−1 (low dose) for 120 min to measure the insulin sensitivity of EGP, glycerol Ra and palmitic acid Ra; step two consisted of insulin infusion at 1.5 mU kg−1 min−1 (high dose) for 180 min to measure the insulin sensitivity of glucose uptake [21]. Glucose concentration was maintained at euglycaemia using dextrose spiked with [6,6-2H2]glucose (8 mg/g glucose for step 1 and 10 mg/g glucose for step 2). Blood samples were taken every 30 min except between 210 and 240 min (low-dose steady state), and between 390 and 420 min (high-dose steady state) when samples were taken every 5 min. Glucagon samples were taken at the end of each steady state.
Body composition and intracellular fat measurements
Height, weight and WHR were measured before each metabolic study. IHCL and IMCL were measured by 1H-MRS within 1 week of the metabolic study and prior to the start of the exercise programme [22, 23] as detailed in ESM 1. Subjects were asked not to exercise for 72 h prior to the measurement. The measurements were repeated under the same conditions after the 6-week exercise or control period [24]. 1H-MRS could not be performed on one of the subjects in the control group due to metal implants and on one of the subjects in the exercise group due to technical problems with the scanner.
Computer tomography
Computer tomography (CT) was used to measure cross-sectional abdominal visceral adipose tissue areas at L4/5 region and the cross-sectional muscle and adipose tissue areas of both legs at the mid-thigh region [25], as detailed in ESM 1. In three subjects subcutaneous abdominal fat could not be measured due to insufficient data collection.
Analytical methods
Glucose, glycerol and palmitic acid isotopic enrichments and concentration of palmitic acid were measured by GC-MS on a HP 5971A MSD (Agilent Technologies, Wokingham, Berks, UK) according to methods previously described [20, 21, 26] as detailed in ESM 1. Glycerol and glucose concentrations were also determined as detailed in ESM 1.
Insulin was measured using an in-house double antibody RIA [27] (intra-assay CV 6%). Plasma adiponectin was measured by RIA using commercially available kits (Linco Research, St Louis, MO, USA; intra-assay CV 5%). Serum NEFA concentration was determined using a commercially available kit (Wako Chemicals, Neuss, Germany; inter-assay CV 3.0%).
Calculations
EGP and glucose uptake were calculated using non-steady-state equations proposed by Steele and modified for stable isotopes [28]. The volume of distribution was assumed to be 22% of body weight. The calculation was also modified for inclusion of [6,6-2H2]glucose in the dextrose infusion [29]. Prior to calculation of glucose turnover, plasma glucose concentration and glucose enrichment time courses were smoothed using optimal segments technique analysis [30]. EGP was calculated at basal and following low-dose insulin. Glucose uptake and metabolic clearance rate (MCR) were calculated at basal and following high-dose insulin. Glucose MCR was calculated from glucose uptake/glucose concentration. Glycerol and palmitic acid Ra and uptake were also calculated using the non-steady-state Steele equations modified for stable isotopes at basal and following low-dose insulin. The volume of distribution for glycerol and palmitic acid were assumed to be 230 and 40 ml/kg respectively [19]. Insulin MCR at each steady state for the low-dose and high-dose insulin was calculated as the insulin infusion rate/insulin concentration.
Statistical analysis
The results are presented as means ± SE. Baseline characteristics of the two groups at 0 week and changes in measurements between baseline and 6 weeks in the two groups were compared using Student’s t tests. Repeated-measurements ANOVA followed by Bonferroni’s multiple comparison test were used for statistical comparisons within the time courses of the experiments for insulin and glucose concentrations. Correlation for log adiponectin concentration with log IHCL content was made in SPSS (SPSS 12.0.2 for Windows; SPSS, Chicago, IL, USA) using Pearson’s correlation. Within-group changes between baseline and 6 weeks were compared using paired t tests. Intracellular fat measurements were analysed with a Mann–Whitney U test. p values <0.05 were considered statistically significant.
Results
Body composition and hormones
Subjects in the exercise group did not differ from those in the control group with regard to age, BMI, WHR, fitness level (in terms of VO2max) and lipid profile at 0 week (Table 1). In the exercise group VO2max increased by 20 ± 5% after 6 weeks with no change in the control group (Δ control vs Δ exercise, p < 0.001) (Table 1). There was a 3 ± 1% decrease in WHR in the exercise group, with no change in the control group (Δ control vs Δ exercise, p = 0.03) (Table 1). There was no significant change in body weight, BMI, or lipid profile in either group at 6 weeks. There was no significant difference in the change in adiponectin between 0 and 6 weeks in the two groups (Table 1).
At 0 week there was no difference in intra-abdominal and subcutaneous abdominal adipose tissue, leg subcutaneous adipose tissue and leg muscle as calculated from the CT scans (Table 2). After 6 weeks leg muscle was increased by 3.7 ± 1% in the exercise group (p = 0.006), but the change between groups was not significant (p = 0.06). There was a significant within-group decrease in visceral fat in the exercise group (p = 0.034), but the change in visceral fat between groups was not significant. No significant change in leg fat or subcutaneous abdominal fat was seen in either group after 6 weeks.
IHCL and IMCL content of tibialis anterior muscle did not differ between groups at 0 week (Table 2). After 6 weeks, IHCL and IMCL contents did not change in either group (Table 2). Fasting log adiponectin concentration in all subjects at 0 week correlated inversely with log IHCL content (r = −0.732, p = 0.001).
Hyperinsulinaemic–euglycaemic clamp: EGP, glucose uptake and MCR
Plasma glucose concentration at basal and during the hyperinsulinaemic–euglycaemic clamp with low-dose and high-dose insulin was not different within or between groups at 0 and 6 weeks (Fig. 1). Serum insulin concentration was not different within or between groups at 0 and 6 weeks at basal, during low-dose infusion and during high-dose infusion (Fig. 1).
Glucose (a) and insulin concentrations (b) in the exercise (blue line) and control groups (red line) at 0 week (dotted line ●) and 6 weeks (solid line ▲). There were no differences within or between groups in glucose or insulin concentrations, either at basal, or following the low-dose (LD) and high-dose (HD) insulin infusions.
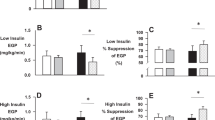
No difference was seen in basal EGP between groups at 0 week (control group, 8.1 ± 0.5 μmol kg−1 min−1; exercise group, 7.8 ± 0.5 μmol kg−1 min−1) or 6 weeks (control group, 7.5 ± 0.7 μmol kg−1 min−1; exercise group, 7.5 ± 1.2 μmol kg−1 min−1). Following low-dose insulin, EGP was lower in the exercise group at 6 weeks (2.3 ± 0.4 μmol kg−1 min−1) than 0 week (3.4 ± 0.4 μmol kg−1 min−1) (p = 0.026). There was no change in the control group (0 week 3.1 ± 0.7 μmol kg−1 min−1; 6 weeks; 3.2 ± 0.4 μmol kg−1 min−1). The change in EGP with low-dose insulin was significantly different between groups (Δ control vs Δ exercise, p = 0.014) (Fig. 2a).
Rates of a EGP, b glucose uptake (Rd), and c glucose MCR during basal and low-dose (LD) or high-dose (HD) insulin infusion in the control group (white bars) and exercise (exe) group (grey bars) at 0 week (open bars) and 6 weeks (hatched bars). *p < 0.03 for 0 week vs 6 weeks; **p < 0.01 for 0 week vs 6 weeks; †p < 0.04 for Δ control vs Δ exe; ‡p = 0.053 for glucose uptake). For actual p values refer to the text
After 6 weeks, the glucose uptake following high-dose insulin infusion (52.7 ± 3.7 μmol kg−1 min−1) was significantly greater in the exercise group than at 0 week (45.1 ± 3.7 μmol kg−1 min−1) (p = 0.007). This change in glucose uptake was greater than in the control group (45.3 ± 3.0 μmol kg−1 min−1, 0 week vs 46.2 ± 3.3 μmol kg−1 min−1, 6 weeks), but did not achieve statistical significance (Δ control vs Δ exercise, p = 0.053) (Fig. 2b).
After 6 weeks, the glucose infusion rate following high-dose insulin infusion (53.1 ± 3.2 μmol kg−1 min−1) was significantly greater in the exercise group than at 0 week (46.3 ± 3.7 μmol kg−1 min−1) (p = 0.016). This change in glucose infusion rate was greater than in the control group (42.8 ± 4.4 μmol kg−1 min−1, 0 week vs 44.2 ± 3.8 μmol kg−1 min−1, 6 weeks), but did not achieve statistical significance (Δ control vs Δ exercise, p = 0.08).
Basal glucose MCR was not different between groups at 0 week (control subjects, 1.8 ± 0.1 ml kg−1 min−1; exercise group, 1.8 ± 0.1 ml kg−1 min−1) or 6 weeks (control subjects, 1.6 ± 0.1 ml kg−1 min−1; exercise group, 1.8 ± 0.1 ml kg−1 min−1). After 6 weeks the change in glucose MCR following high-dose insulin infusion in the exercise group (11.8 ± 0.8 ml kg−1 min−1 vs 10.2 ± 0.8 ml kg−1 min−1, 0 week) was significant (p = 0.04). This change in glucose MCR was greater than in the control group (0 week, 9.4 ± 0.9 ml kg−1 min−1; 6 weeks, 8.6 ± 0.5 ml kg−1 min−1) (Δ control vs Δ exercise, p = 0.048) (Fig. 2c).
NEFA, palmitic acid and glycerol concentration and Ra
As shown in Table 3 and Fig. 3, basal NEFA, palmitic acid and glycerol concentration and basal palmitic acid and glycerol Ra and uptake at 0 week were not different between the two groups. After 6 weeks, basal NEFA and glycerol concentration decreased in the exercise group (both p = 0.005), with no change in the control group. The change in basal NEFA and glycerol concentration in the exercise group was significantly different from the control group (Δ control vs Δ exercise, p = 0.004 and p = 0.021, respectively). Basal palmitic acid concentration was lower after 6 weeks exercise, but this did not achieve statistical significance when compared with 0 week. When compared with the control group, the change in palmitic acid concentration was significantly different between groups (Δ control vs Δ exercise, p = 0.018).
Glycerol (a) and palmitic acid (b) Ra, and NEFA concentration (c) during basal and low-dose (LD) insulin infusion in the control (white bars) and exercise (exe) groups (grey bars) at 0 week (open bars) and 6 weeks (hatched bars). *p < 0.04 for 0 week vs 6 weeks; **p < 0.01 for 0 week vs 6 weeks; †p < 0.04 for Δ control vs Δ exe; and ‡p < 0.01 for Δ control vs Δ exe. For actual p values refer to the text
Basal glycerol Ra (3.0 ± 0.49 μmol kg−1 min−1) and palmitic acid Ra (1.5 ± 0.1 μmol kg−1 min−1) were both lower after 6 weeks in the exercise group (2.3 ± 0.36 μmol kg−1 min−1, p = 0.003 and 1.23 ± 0.16 μmol kg−1 min−1, p = 0.042, respectively) with no change in these measurements in the control group (glycerol Ra, 0 week, 3.0 ± 0.45 μmol kg−1 min−1 vs 6 weeks, 3.0 ± 0.44 μmol kg−1 min−1; palmitic acid Ra, 0 week, 1.22 ± 0.10 μmol kg−1 min−1 vs 6 weeks 1.36 ± 0.13 μmol kg−1 min−1, respectively) (Fig. 3). Following low-dose insulin, palmitic acid Ra was lower at 6 weeks than 0 week in the exercise group, but this did not achieve statistical significance. The change in palmitic acid Ra with low-dose insulin was significantly different from the control group (Δ control vs Δ exercise p = 0.028). Glycerol Ra was not different following low-dose insulin after 6 weeks exercise.
Palmitic acid and glycerol uptake followed the same pattern as palmitic acid and glycerol Ra in both groups at basal and after the low-dose insulin infusion. Basal glycerol uptake (3.0 ± 0.54 μmol kg−1 min−1) and palmitic acid uptake (1.55 ± 0.08 μmol kg−1 min−1) were both lower after 6 weeks in the exercise group (2.3 ± 0.39 μmol kg−1 min−1, p = 0.012 and 1.26 ± 0.13 μmol kg−1 min−1, p = 0.042, respectively), with no change in these measurements in the control group (glycerol uptake; 0 week, 3.08 ± 0.51 μmol kg−1 min−1 vs 6 weeks, 3.07 ± 0.48 μmol kg−1 min−1; palmitic acid uptake, 0 week, 1.27 ± 0.10 μmol kg−1 min−1 vs 6 weeks 1.39 ± 0.12 μmol kg−1 min−1, respectively).
Discussion
Using a two-step euglycaemic–hyperinsulinaemic clamp, we demonstrated that 6 weeks of exercise training in sedentary male subjects resulted in increased insulin sensitivity of both EGP and peripheral glucose uptake. We also demonstrated that exercise training resulted in decreased fasting palmitic acid Ra, glycerol Ra and NEFA concentrations in the absence of any change in insulin concentrations, suggesting an increase in the insulin sensitivity of lipolysis.
The improvement of insulin sensitivity of EGP that is effected by chronic exercise training has not been previously demonstrated. Although DeFronzo et al. reported that a 6-week exercise training programme in moderately obese subjects improved the insulin sensitivity of EGP measured with a hyperinsulinaemic–euglycaemic clamp (1 mU kg−1 min−1) [31], the post-training assessments were made 48 h after the last exercise bout. It has been shown that the acute effect of exercise on insulin sensitivity is maintained for 48 h, but is unmeasurable after 72 h [32]. In the current study all subjects were studied 72 h after the last exercise session. DiPietro et al. [33] found no difference in the insulin sensitivity of EGP after 9 months of exercise training at moderate or high intensity in older non-obese women studied 72 h after the final exercise bout and compared to a control group. This may be due to the lack of change in fitness, as VO2max did not change with exercise training. In the current study there was a significant improvement in VO2max following exercise training.
We also demonstrated that after exercise training glucose uptake and clearance increased with the high-dose insulin infusion in agreement with a previous study showing an improvement in peripheral insulin sensitivity following exercise training in insulin-resistant men, who were studied 96 h after the last exercise session [34].
We hypothesised that an improvement in insulin sensitivity after exercise training may be explained by adaptive changes in fatty acid metabolism, changes in visceral fat or changes in liver and muscle triacylglycerol content. We measured lipolysis in the fasting state and following low-dose insulin using isotopic tracers of both glycerol and palmitic acid. Whole-body glycerol Ra has been used in many studies to provide an estimate of the rate of lipolysis. However, this may overestimate lipolysis, since VLDL hydrolysis will contribute to glycerol Ra. Although this will be a small percentage of total glycerol Ra in the fasting state, during an hyperinsulinaemic clamp this contribution may increase, since insulin increases lipoprotein lipase mass and activity [35, 36]. Measurement of NEFA Ra using an isotope of one of the more abundant circulating fatty acids such as palmitic acid or oleic acid has also been used as a measure of whole-body lipolysis, although this may lead to an underestimation of lipolysis due to intracellular recycling of fatty acids into triacylglycerol. Both fasting glycerol Ra and palmitic acid Ra were reduced following an exercise programme in the current study, suggesting a decrease in fasting lipolysis. Since fasting insulin concentrations were not different after exercise training, an increase in the insulin sensitivity of lipolysis in the fasting state seems plausible. This supports the findings of a study that showed a decrease in lipolysis and hormone sensitive lipase activity in isolated adipocytes following endurance training for 4 months in obese subjects [37]. The suppression of NEFA concentration was significantly greater during the low-dose insulin infusion after 6 weeks exercise training, and although the suppression of glycerol and palmitic acid Ra was not different within groups, the decrease in palmitic acid Ra with low-dose insulin in the exercise group was significantly greater than in the control group. This also suggests an improvement in the insulin sensitivity of lipolysis.
The decrease in circulating NEFA may provide a mechanism for the improved insulin sensitivity of EGP and glucose uptake. Previous studies in obese subjects have shown that the insulin sensitivity of glucose production is reduced in the presence of elevated plasma NEFA concentrations resulting from an infusion of intralipid and heparin [38]. In type 2 diabetic subjects reduction of NEFA by administration of Acipimox 120 min before a euglycaemic–hyperinsulinaemic clamp study (108 pmol m−2 min−1; 0.4 mU kg−1 min−1) was shown to reduce EGP [39], while in vitro increased NEFA levels have been shown to impair insulin binding and clearance in hepatocytes [40]. NEFA have also been shown to reduce insulin-stimulated glucose transport and whole-body glucose clearance in healthy subjects [41], while overnight administration of Acipimox has been shown to reduce NEFA and improve insulin sensitivity of glucose uptake in obese subjects [42]. Other factors may also contribute to the improvement of insulin sensitivity of peripheral glucose uptake, such as the increase in muscle mass.
The decrease in visceral fat in the exercise group may also have contributed to the improvement in insulin sensitivity of EGP. Venous drainage from visceral fat enters the hepatic portal circulation, thus NEFA released from visceral fat will reach the liver directly. Nielsen et al. measured the contribution of splanchnic lipolysis to hepatic NEFA delivery in lean and obese men and women, showing that this ranges from less than 10% to almost 50% and increases as a function of visceral fat [2]. As only a small percentage of NEFA from visceral fat reach the systemic circulation, a decrease in visceral fat would not be expected to affect systemic NEFA levels or palmitic acid Ra. The subcutaneous fat depot is thus largely responsible for the release of NEFA into the systemic circulation. We observed that there was no change in subcutaneous fat (measured by CT scanning of leg fat and abdominal subcutaneous fat), following the exercise programme, a finding that suggests that the decrease in lipolysis in the fasting state was not due to a decrease in subcutaneous fat mass.
Recent studies suggest that hepatic triacylglycerol content may be the main determinant of hepatic insulin resistance. Hepatic triacylglycerol content measured with proton spectroscopy is closely correlated with hepatic insulin sensitivity in type 2 diabetic patients [43] and in non-diabetic men [13]. In the latter study, this was independent of intra-abdominal obesity measured by MRI and of overall obesity, whereas a subsequent study in patients with type 2 diabetes found liver triacylglycerol to be closely related to visceral fat measured by CT [44]. In the current study, the increase in the insulin sensitivity of EGP occurred in the absence of any significant change in IHCL. However, there was a large variance in IHCL and the study may have been not sufficiently powered to detect any significant change.
Intracellular muscle triacylglycerol deposition has also been shown to retard GLUT4 trafficking, thereby reducing glucose entry into cells [8]. Reduction in muscle triacylglycerol by dietary restriction has been shown to improve muscle glucose uptake, but in the present study the exercise programme did not affect IMCL, suggesting the relationship between IMCL and insulin sensitivity may also reflect other factors [45].
In summary, this study suggests that an improvement in the insulin sensitivity of lipolysis, resulting in decreased availability of circulating NEFA, may contribute to the observed improvement in hepatic insulin sensitivity of EGP and peripheral insulin sensitivity of glucose uptake following an exercise programme. While it is well recognised that insulin sensitivity is improved following exercise training in patients with type 2 diabetes and obesity, this is the first study to show that the chronic effects of exercise training (>72 h after the last bout of exercise) results in improved insulin sensitivity of whole-body lipolysis in the fasting state and of endogenous glucose production rate during a euglycaemic–hyperinsulinaemic clamp.
Abbreviations
- CT:
-
computer tomography
- EGP:
-
endogenous glucose production
- 1H-MRS:
-
1H-magnetic resonance spectroscopy
- IHCL:
-
intrahepatocellular lipid
- IMCL:
-
intramyocellular lipid
- MCR:
-
metabolic clearance rate
- Production rate:
-
Ra
- VO2max :
-
maximal aerobic capacity
References
Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S (1998) The metabolically obese, normal-weight individual revisited. Diabetes 47:699–713
Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD (2004) Splanchnic lipolysis in human obesity. J Clin Invest 113:1582–1588
Horowitz JF, Coppack SW, Klein S (2001) Whole-body and adipose tissue glucose metabolism in response to short-term fasting in lean and obese women. Am J Clin Nutr 73:517–522
Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM (1989) Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest 83:1168–1173
Boden G (1998) Free fatty acids (FFA), a link between obesity and insulin resistance. Front Biosci 3:D169–D175
Randle PJ, Garland PB, Hales CN, Newsholme EA (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1:785–789
Roden M, Krssak M, Stingl H et al (1999) Rapid impairment of skeletal muscle glucose transport/phosphorylation by free fatty acids in humans. Diabetes 48:358–364
Dresner A, Laurent D, Marcucci M et al (1999) Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 103:253–259
Wiesenthal SR, Sandhu H, McCall RH et al (1999) Free fatty acids impair hepatic insulin extraction in vivo. Diabetes 48:766–774
Griffin ME, Marcucci MJ, Cline GW et al (1999) Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signalling cascade. Diabetes 48:1270–1274
Stein DT, Esser V, Stevenson BE et al (1996) Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest 97:2728–2735
Virkamaki A, Korsheninnikova E, Seppala-Lindroos A et al (2001) Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes 50:2337–2343
Seppala-Lindroos A, Vehkavaara S, Hakkinen AM et al (2002) Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 87:3023–3028
Perseghin G, Price TB, Petersen KF et al (1996) Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 335:1357–1362
Boden G (1997) Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46:3–10
Meek SE, Nair KS, Jensen MD (1999) Insulin regulation of regional free fatty acid metabolism. Diabetes 48:10–14
Borg G, Linderholm H (1967) Perceived exertion and pulse rate during graded exercise in various age groups. Acta Med Scand (Suppl) 472:194–206
Eriksson J, Taimela S, Koivisto VA (1997) Exercise and the metabolic syndrome. Diabetologia 40:125–135
Romijn JA, Coyle EF, Sidossis LS et al (1993) Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol 265:E380–E391
Gibney J, Healy ML, Stolinski M et al (2003) Effect of growth hormone (GH) on glycerol and free fatty acid metabolism during exhaustive exercise in GH-deficient adults. J Clin Endocrinol Metab 88:1792–1797
Brackenridge A, Pearson ER, Shojaee-Moradie F, Hattersley AT, Russell-Jones D, Umpleby AM (2006) Contrasting insulin sensitivity of endogenous glucose production rate in subjects with hepatocyte nuclear factor-1beta and-1alpha mutations. Diabetes 55:405–411
Rico-Sanz J, Hajnal JV, Thomas EL, Mierisova S, Ala-Korpela M, Bell JD (1998) Intracellular and extracellular skeletal muscle triglyceride metabolism during alternating intensity exercise in humans. J Physiol 510:615–622
Rico-Sanz J, Thomas EL, Jenkinson G, Mierisova S, Iles R, Bell JD (1999) Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by (1)H-MRS. J Appl Physiol 87:2068–2072
Szczepaniak LS, Nurenberg P, Leonard D et al (2005) Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 288:E462–E468
Rendell M, Hulthen UL, Tornquist C, Groop L, Mattiasson I (2001) Relationship between abdominal fat compartments and glucose and lipid metabolism in early postmenopausal women. J Clin Endocrinol Metab 86:744–749
Shojaee-Moradie F, Jackson NC, Jones RH, Mallet AI, Hovorka R, Umpleby AM (1996) Quantitative measurement of 3-O-methyl-d-glucose by gas chromatography-mass spectrometry as a measure of glucose transport in vivo. J Mass Spectrom 31:961–966
Sonksen PH (1976) Double antibody technique for the simultaneous assay of insulin and growth hormone. In: Antoniades HN (ed) Hormones in human blood: detection and assay. Harvard University Press, Cambridge, MA, pp 176–199
Steele R, Bishop JS, Dunn A, Altszuler N, Rathbeb I, Debodo RC (1965) Inhjbition by insulin of hepatic glucose production in the normal dog. Am J Physiol 208:301–306
Finegood DT, Bergman RN, Vranic M (1987) Estimation of endogenous glucose production during hyperinsulinemic–euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes 36:914–924
Finegood DT, Bergman RN (1983) Optimal segments: a method for smoothing tracer data to calculate metabolic fluxes. Am J Physiol 244:E472–E479
DeFronzo RA, Sherwin RS, Kraemer N (1987) Effect of physical training on insulin action in obesity. Diabetes 36:1379–1385
Eriksson J, Taimela S, Koivisto VA (1997) Exercise and the metabolic syndrome. Diabetologia 40:125–135
DiPietro L, Dziura J, Yeckel CW, Neufer PD (2006) Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J Appl Physiol 100:142–149
Hughes VA, Fiatarone MA, Fielding RA et al (1993) Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol 264:E855–E862
Eckel RH, Yost TJ, Jensen DR (1995) Alterations in lipoprotein lipase in insulin resistance. Int J Obes Relat Metab Disord 19(Suppl 1):S16–S21
Miyashita Y, Ebisuno M, Ohhira M et al (2006) Enhancement of serum lipoprotein lipase mass levels by intensive insulin therapy. Diabetes Res Clin Pract 72:61–67
De Glisezinski I, Crampes F, Harant I et al (1998) Endurance training changes in lipolytic responsiveness of obese adipose tissue. Am J Physiol 275:E951–E956
Bevilacqua S, Bonadonna R, Buzzigoli G et al (1987) Acute elevation of free fatty acid levels leads to hepatic insulin resistance in obese subjects. Metabolism 36:502–506
Saloranta C, Franssila-Kallunki A, Ekstrand A, Taskinen MR, Groop L (1991) Modulation of hepatic glucose production by non-esterified fatty acids in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 34:409–415
Svedberg J, Bjorntorp P, Smith U, Lonnroth P (1990) Free-fatty acid inhibition of insulin binding, degradation, and action in isolated rat hepatocytes. Diabetes 39:570–574
Krebs M, Krssak M, Nowotny P et al (2001) Free fatty acids inhibit the glucose-stimulated increase of intramuscular glucose-6-phosphate concentration in humans. J Clin Endocrinol Metab 86:2153–2160
Santomauro AT, Boden G, Silva ME et al (1999) Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 48:1836–1841
Ryysy L, Hakkinen AM, Goto T et al (2000) Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes 49:749–758
Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC (2003) Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab 285:E906–E916
Frost GS, Goff LM, Hamilton G et al (2003) Carbohydrate-induced manipulation of insulin sensitivity independently of intramyocellular lipids. Br J Nutr 89:365–375
Acknowledgements
This study was funded by the British Heart Foundation (PG/2000056). We are grateful for the technical assistance of P. Croos and W. Jefferson. We would also like to thank N. Patel and G. Hamilton for their help with this project.
Duality of interest
The authors state that there is no duality of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shojaee-Moradie, F., Baynes, K.C.R., Pentecost, C. et al. Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia 50, 404–413 (2007). https://doi.org/10.1007/s00125-006-0498-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-006-0498-7