Abstract
Purpose
Eliciting antitumor T-cell response by targeting the PD-1/PD-L1 axis with checkpoint inhibitors has emerged as a novel therapeutic strategy in non-small cell lung cancer (NSCLC). The identification of predictors for sensitivity or resistance to these agents is, therefore, needed. Herein, we investigate the correlation of metabolic information on FDG-PET with tissue expression of immune-checkpoints and other markers of tumor-related immunity in resected NSCLC patients.
Materials and methods
All patients referred to our institution for upfront surgical resection of NSCLC, who were investigated with FDG-PET prior to surgery, were consecutively included in the study. From January 2010 to May 2014, 55 patients (stage IA-IIIB; M:F = 42:13; mean age 68.9 years) were investigated. Sampled surgical tumor specimens were analyzed by immunohistochemistry (IHC) for CD68-TAMs (tumor-associated macrophages), CD8-TILs (tumor infiltrating lymphocytes), PD-1-TILs, and PD-L1 tumor expression. Immunoreactivity was evaluated, and scores were compared with imaging findings. FDG-PET images were analyzed to define semi-quantitative parameters: SUVmax and SUVmean. Metabolic information on FDG-PET was correlated with tissue markers expression and disease-free survival (DFS) considering a median follow-up of 16.2 months.
Results
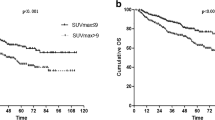
Thirty-six adenocarcinomas (ADC), 18 squamous cell carcinomas (SCC), and one sarcomatoid carcinoma were analyzed. All tumors resulted positive at FDG-PET: median SUVmax 11.3 (range: 2.3–32.5) and SUVmean 6.4 (range: 1.5–13) both resulted significantly higher in SCC compared to other NSCLC histotypes (p = 0.007 and 0.048, respectively). IHC demonstrated a median immunoreactive surface covered by CD68-TAMs of 5.41 % (range: 0.84–14.01 %), CD8-TILs of 2.9 % (range: 0.11–11.92 %), PD-1 of 0.65 % (range: 0.02–5.87 %), and PD-L1 of 0.7 % (range: 0.03–10.29 %). We found a statistically significant correlation between SUVmax and SUVmean with the expression of CD8 TILs (rho = 0.31; p = 0.027) and PD-1 (rho = 0.33; p = 0.017 and rho = 0.36; p = 0.009, respectively). The other tissue markers correlated as follows: CD8 TILs and PD-1 (rho = 0.45; p = 0.001), CD8 TILs and PD-L1 (rho = 0.41; p = 0.003), CD68-TAMs and PD-L1 (rho = 0.30; p = 0.027), PD-1 and PD-L1 (rho = 0.26; p = 0.059). With respect to patients’ outcome, SUVmax, SUVmean, and disease stage showed a statistically significant correlation with DFS (p = 0.002, 0.004, and <0.001, respectively).
Conclusions
The present study shows a direct association between metabolic parameters on FDG-PET and the expression of tumor-related immunity markers, suggesting a potential role for FDG-PET to characterize the tumor microenvironment and select NSCLC patients candidate to checkpoint inhibitors.




Similar content being viewed by others
References
Saintigny P, Burger JA. Recent advances in non-small cell lung cancer biology and clinical management. Discov Med. 2012;13:287–97.
Xia B, Herbst RS. Immune checkpoint therapy for non-small-cell lung cancer: an update. Immunotherapy. 2016;8:279–98.
Bremnes RM, Busund LT, Kivaer TL, et al. The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non-small cell lung cancer. J Thorac Oncol. 2016. doi:10.1016/j.jtho.2016.01.015.
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22.
Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22.
Vesery MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71.
Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61.
Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4.
Restifo NP. A “big data” view of the tumor “immunome”. Immunity. 2013;39:631–2.
Whiteside TL. Immune responses to cancer: are they potential biomarkers of prognosis? Front Oncol. 2013;3:3–107.
Remark R, Becker C, Gomez JE, et al. The non-small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome. Am J Respir Crit Care Med. 2015;191:377–90.
Sundar R, Soong R, Byoung-Chul C, et al. Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer. 2014;85:101–9.
Grosso J, Horak CE, Inzunza D, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538). J Clin Oncol. 2013;31 (suppl:abstract 3016).
D’Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112:95–102.
He J, Hu Y, Hu M, Li B. Development of PD-1/PD-L1 pathway in tumor immune microenvironment and treatment for non-small cell lung cancer. Sci Rep. 2015;5:13110.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35.
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;37:1627–39.
Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomized controlled trial. Lancet. 2015. doi:10.1016/S0140-6736(15)01281-7.
Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016. doi:10.1016/S0140-6736(16)00587-0.
Soria JC, Marabelle A, Brahmer JR, et al. Immune checkpoint modulation for non-small cell lung cancer. Clin Cancer Res. 2015;21:2256–62.
Takeuchi S, Khiewvan B, Fox PS, et al. Impact of initial PET/CT staging in terms of clinical stage, management plan, and prognosis in 592 patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2014;41:906–14.
Liao S, Penney BC, Wroblewski K, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012;39:27–38.
Palsson-McDermott EM, O’Neill LAJ. The Warburg effect then and now: from cancer to inflammatory diseases. BioEssays. 2013;35:965–73.
Appelberg R, Moreira D, Barriera-Silva P, et al. The Warburg effect in mycobacterial granulomas is dependent on the recruitment and activation of macrophages by interferon-y. Immunology. 2015;145:498–507.
Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–43.
Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–41.
Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Castino GF, Cortese N, Capretti G, et al. Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma. OncoImmunology. 2015. doi:10.1080/2162402X.2015.1085147.
Vansteenkiste JF, Cho B, Vanakesa T, et al. MAGRIT, a double-blind randomized, placebo-controlled phase III study to assess the efficacy of recMAGE-A3 + AS15 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small cell lung cancer. Ann Oncol. 2014. doi:10.1093/annonc/mdu347.
Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomized, double-blind, phase 3 trial. Lancet Oncol. 2014;15:59–68.
Heskamp S, Hobo W, Molkenboer-Kuenen JD, et al. Noninvasive imaging of tumor PD-L1 expression using radiolabeled anti-PD-L1 antibodies. Cancer Res. 2015;75:2928–36.
Josefsson A, Nedrow JR, Parks S, et al. Imaging, Biodistribution, and Dosimetry of radionuclide-labeled PD-L1 antibody in an immunocompetent mouse model of breast cancer. Cancer Res. 2016;76:472–9.
Tavarè R, Escuin-Ordinas H, Mok S, et al. An effective immune-PET imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res. 2016;76:73–82.
Liu J, Dong M, Sun X, et al. Prognostic value of 18F-FDG PET/CT in surgical non-small cell lung cancer: a meta-analysis. PLoS One. 2016;11:e0146195.
Kurtupek E, Cayci M, Duzgun N, et al. (18)F-FDG PET/CT mean SUV and metabolic tumor volume for mean survival time in non-small cell lung cancer. Clin Nucl Med. 2015;40:459–63.
Schmidt LH, Kummel A, Gorlich D, et al. PD-1 and PD-L1 expression in NSCLC indicate a favourable prognosis in defined subgroups. PLoS One. 2015;10:e0136023.
Schalper KA, Brown J, Carvajal-Hausdorf D, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst. 2015;107(3):dju435.
Wang A, Wang HY, Liu Y, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol. 2015;41:450–6.
Horne ZD, Jack R, Gray ZT, et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res. 2011;171:1–5.
Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74.
Hersbt RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;51:563–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study herein presented was approved by the local review board and performed in accordance with the principles of good clinical practice, with the Declaration of Helsinki, and with the national regulations regarding clinical trials. Informed consent was obtained for all patients or their legal guardians, and patient assent was obtained whenever appropriate.
Conflicts of interest
The authors have declared no conflicts of interest.
Additional information
Egesta Lopci and Luca Toschi contributed equally to this work.
Arturo Chiti and Federica Marchesi can be considered as co-last authors.
Rights and permissions
About this article
Cite this article
Lopci, E., Toschi, L., Grizzi, F. et al. Correlation of metabolic information on FDG-PET with tissue expression of immune markers in patients with non-small cell lung cancer (NSCLC) who are candidates for upfront surgery. Eur J Nucl Med Mol Imaging 43, 1954–1961 (2016). https://doi.org/10.1007/s00259-016-3425-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3425-2