Abstract
Introduction
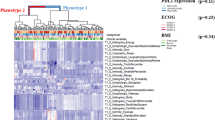
Immunotherapy has improved outcomes for patients with non-small cell lung cancer (NSCLC), yet durable clinical benefit (DCB) is experienced in only a fraction of patients. Here, we test the hypothesis that radiomics features from baseline pretreatment 18F-FDG PET/CT scans can predict clinical outcomes of NSCLC patients treated with checkpoint blockade immunotherapy.
Methods
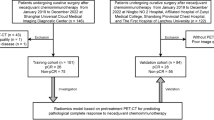
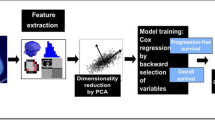
This study included 194 patients with histologically confirmed stage IIIB-IV NSCLC with pretreatment PET/CT images. Radiomics features were extracted from PET, CT, and PET+CT fusion images based on minimum Kullback–Leibler divergence (KLD) criteria. The radiomics features from 99 retrospective patients were used to train a multiparametric radiomics signature (mpRS) to predict DCB using an improved least absolute shrinkage and selection operator (LASSO) method, which was subsequently validated in both retrospective (N = 47) and prospective test cohorts (N = 48). Using these cohorts, the mpRS was also used to predict progression-free survival (PFS) and overall survival (OS) by training nomogram models using multivariable Cox regression analyses with additional clinical characteristics incorporated.
Results
The mpRS could predict patients who will receive DCB, with areas under receiver operating characteristic curves (AUCs) of 0.86 (95%CI 0.79–0.94), 0.83 (95%CI 0.71–0.94), and 0.81 (95%CI 0.68–0.92) in the training, retrospective test, and prospective test cohorts, respectively. In the same three cohorts, respectively, nomogram models achieved C-indices of 0.74 (95%CI 0.68–0.80), 0.74 (95%CI 0.66–0.82), and 0.77 (95%CI 0.69–0.84) to predict PFS and C-indices of 0.83 (95%CI 0.77–0.88), 0.83 (95%CI 0.71–0.94), and 0.80 (95%CI 0.69–0.91) to predict OS.
Conclusion
PET/CT-based signature can be used prior to initiation of immunotherapy to identify NSCLC patients most likely to benefit from immunotherapy. As such, these data may be leveraged to improve more precise and individualized decision support in the treatment of patients with advanced NSCLC.






Similar content being viewed by others
References
Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Lung cancer and personalized medicine. Springer; 2016. p. 1–19.
Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, et al. Tracking the evolution of non–small-cell lung cancer. N Engl J Med. 2017;376(22):2109–21.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. https://doi.org/10.3322/caac.21442.
Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–65.
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. New Engl J Med. 2016;375(19):1823–33.
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. New Engl J Med. 2015;373(2):123–35. https://doi.org/10.1056/NEJMoa1504627.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. New Engl J Med. 2015;372(21):2018–28.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. New Engl J Med. 2012;366(26):2443–54.
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, Von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65.
Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563.
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. New Engl J Med. 2018;378(22):2078–92. https://doi.org/10.1056/NEJMoa1801005.
Meng X, Huang Z, Teng F, Xing L, Yu J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. 2015;41(10):868–76.
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348(6230):124–8.
Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006.
Beukinga RJ, Hulshoff JB, Mul VE, Noordzij W, Kats-Ugurlu G, Slart RH, et al. Prediction of response to neoadjuvant chemotherapy and radiation therapy with baseline and restaging 18F-FDG PET imaging biomarkers in patients with esophageal cancer. Radiology. 2018;287(3):983–92 172229.
Schwarzenberg J, Czernin J, Cloughesy TF, Ellingson BM, Pope WB, Grogan T, et al. Treatment response evaluation using <sup>18</sup>F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res. 2014;20(13):3550–9. https://doi.org/10.1158/1078-0432.ccr-13-1440.
Napel S, Mu W, Jardim-Perassi BV, Aerts H, Gillies RJ. Quantitative imaging of cancer in the postgenomic era: radio(geno)mics, deep learning, and habitats. Cancer. 2018;124(24):4633–49. https://doi.org/10.1002/cncr.31630.
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348(6230):124–8. https://doi.org/10.1126/science.aaa1348.
Campesato LF, Barroso-Sousa R, Jimenez L, Correa BR, Sabbaga J, Hoff PM, et al. Comprehensive cancer-gene panels can be used to estimate mutational load and predict clinical benefit to PD-1 blockade in clinical practice. Oncotarget. 2015;6(33):34221–7.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Kullback S, Leibler RA. On information and sufficiency. Ann Math Stat. 1951;22(1):79–86.
Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Ser B Methodol. 1996;58(1):267–88.
Crivellaro C, Signorelli M, Guerra L, De Ponti E, Buda A, Dolci C, et al. 18F-FDG PET/CT can predict nodal metastases but not recurrence in early stage uterine cervical cancer. Gynecol Oncol. 2012;127(1):131–5.
Taniguchi K, Okami J, Kodama K, Higashiyama M, Kato K. Intratumor heterogeneity of epidermal growth factor receptor mutations in lung cancer and its correlation to the response to gefitinib. Cancer Sci. 2008;99(5):929–35.
Fitzgerald M, Saville BR, Lewis RJ. Decision curve analysis. Jama. 2015;313(4):409–10.
Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2(9):683.
Park JE, Kim D, Kim HS, Park SY, Kim JY, Cho SJ, et al. Quality of science and reporting of radiomics in oncologic studies: room for improvement according to radiomics quality score and TRIPOD statement. Eur Radiol. 2019. https://doi.org/10.1007/s00330-019-06360-z.
Wu J, Aguilera T, Shultz D, Gudur M, Rubin DL, Loo BW Jr, et al. Early-stage non–small cell lung cancer: quantitative imaging characteristics of 18F fluorodeoxyglucose PET/CT allow prediction of distant metastasis. Radiology. 2016;281(1):270–8.
Vallières M, Freeman CR, Skamene SR, El Naqa I. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol. 2015;60(14):5471.
Carvalho S, Leijenaar R, Troost E, van Elmpt W, Muratet J-P, Denis F, et al. Early variation of FDG-PET radiomics features in NSCLC is related to overall survival-the “delta radiomics” concept. Radiother Oncol. 2016;118:S20–S1.
Oikonomou A, Khalvati F, Tyrrell PN, Haider MA, Tarique U, Jimenez-Juan L, et al. Radiomics analysis at PET/CT contributes to prognosis of recurrence and survival in lung cancer treated with stereotactic body radiotherapy. Sci Rep. 2018;8(1):4003.
Kirienko M, Cozzi L, Antunovic L, Lozza L, Fogliata A, Voulaz E, et al. Prediction of disease-free survival by the PET/CT radiomic signature in non-small cell lung cancer patients undergoing surgery. Eur J Nucl Med Mol Imaging. 2018;45(2):207–17.
Fukunaga H, Sekimoto M, Ikeda M, Higuchi I, Yasui M, Seshimo I, et al. Fusion image of positron emission tomography and computed tomography for the diagnosis of local recurrence of rectal cancer. Ann Surg Oncol. 2005;12(7):561–9. https://doi.org/10.1245/Aso.2005.08.001.
Nakamoto Y, Senda M, Okada T, Sakamoto S, Saga T, Higashi T, et al. Software-based fusion of PET and CT images for suspected recurrent lung cancer. Mol Imaging Biol. 2008;10(3):147–53. https://doi.org/10.1007/s11307-008-0131-x.
Schaarschmidt BM, Heusch P, Buchbender C, Ruhlmann M, Bergmann C, Ruhlmann V, et al. Locoregional tumour evaluation of squamous cell carcinoma in the head and neck area: a comparison between MRI, PET/CT and integrated PET/MRI. Eur J Nucl Med Mol Imaging. 2016;43(1):92–102. https://doi.org/10.1007/s00259-015-3145-z.
Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med. 2003;44(8):1200–9.
Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–8.
Saeed-Vafa D, Bravo R, Dean JA, El-Kenawi A, Père NM, Strobl M et al. Combining radiomics and mathematical modeling to elucidate mechanisms of resistance to immune checkpoint blockade in non-small cell lung cancer. bioRxiv. 2017:190561.
Longo DL, Bartoli A, Consolino L, Bardini P, Arena F, Schwaiger M, et al. In vivo imaging of tumor metabolism and acidosis by combining PET and MRI-CEST pH imaging. Cancer Res. 2016;76(22):6463–70. https://doi.org/10.1158/0008-5472.CAN-16-0825.
Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, et al. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 2016;76(6):1381–90. https://doi.org/10.1158/0008-5472.CAN-15-1743.
Yip SS, Kim J, Coroller TP, Parmar C, Velazquez ER, Huynh E, et al. Associations between somatic mutations and metabolic imaging phenotypes in non–small cell lung cancer. J Nucl Med. 2017;58(4):569–76.
Kaira K, Higuchi T, Naruse I, Arisaka Y, Tokue A, Altan B, et al. Metabolic activity by 18F–FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging. 2018;45(1):56–66.
Kumar V, Nath K, Berman CG, Kim J, Tanvetyanon T, Chiappori AA, et al. Variance of SUVs for FDG-PET/CT is greater in clinical practice than under ideal study settings. Clin Nucl Med. 2013;38(3):175–82. https://doi.org/10.1097/RLU.0b013e318279ffdf.
Funding
This study was funded by the US Public Health Service research grant U01 CA143062 and R01 CA190105 (awarded to Dr. Gillies).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Robert James Gillies declared a potential conflict with HealthMyne, Inc. (Investor, Board of Advisors). The remaining authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board at the University of South Florida (USF) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The requirement for informed consent was waived.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Advanced Image Analyses (Radiomics and Artificial Intelligence)
Electronic supplementary material
ESM 1
(DOCX 798 kb)
Rights and permissions
About this article
Cite this article
Mu, W., Tunali, I., Gray, J.E. et al. Radiomics of 18F-FDG PET/CT images predicts clinical benefit of advanced NSCLC patients to checkpoint blockade immunotherapy. Eur J Nucl Med Mol Imaging 47, 1168–1182 (2020). https://doi.org/10.1007/s00259-019-04625-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04625-9