Abstract
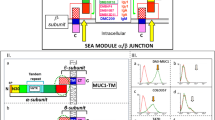
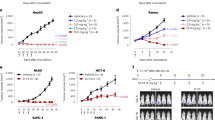
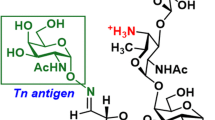
Recently, we described a new carbohydrate-induced conformational tumour-epitope on mucin-1 (MUC1) with the potential for improvement of immunotherapies [29, 30]. PankoMab is a novel antibody, which binds specifically to this epitope and was designed to show the highest glycosylation dependency and the strongest additive binding effect when compared to other MUC1 antibodies. This enables PankoMab to differentiate between tumour MUC1 and non-tumour MUC1 epitopes. It has a high-affinity towards tumour cells (e.g. K D [M] of 0.9 and 3×10−9 towards NM-D4 and ZR75-1, respectively) and detects a very large number of binding sites (e.g. 1.0 and 2.4×106 for NM-D4 and ZR75-1, respectively). PankoMab is rapidly internalised, and after toxin coupling is able to induce very effectively toxin-mediated antigen-specific tumour cell killing. PankoMab reveals a potent tumour-specific antibody-dependent cell cytotoxicity (ADCC). PankoMab is, therefore, distinguished by a combination of advantages compared to other MUC1 antibodies in clinical development, including higher tumour specificity, higher affinity, a higher number of binding sites, largely reduced binding to shed MUC1 from colon and pancreatic carcinoma patients, no binding to mononucleated cells from peripheral blood (except ~7% of activated T cells), stronger ADCC activity and rapid internalisation as required for toxin-mediated cell killing. This renders it a superior antibody for in vivo diagnostics and various immunotherapeutic approaches.





Similar content being viewed by others
Abbreviations
- ADCC:
-
Antibody-dependent cell cytotoxicity
- MUC1:
-
Mucin-1
- PBMC:
-
Peripheral blood mononuclear cells
References
Agrawal B, Krantz MJ, Parker J, Longenecker BM (1998) Expression of MUC1 mucin on activated human T cells: implication for a role of MUC1 in normal immune regulation. Cancer Res 58:4079–4081
Akewanlop C, Watanabe M, Singh B, Walker M, Kufe DW, Hayes DF (2001) Phagocytosis of breast cancer cells mediated by anti-MUC-1 monoclonal antibody, DF3, and its bispecific antibody. Cancer Res 61:4061–4065
Altschuler Y, Kinlough CL, Poland PA, Bruns JB, Apodaca G, Weisz OA, Hughey RP (2000) Clathrin-mediated endocytosis of MUC1 is modulated by its glycosylation state. Mol Biol Cell 11:819–831
Baldus SE, Engelmann K, Hanisch FG (2004) MUC1 and the MUCs: a family of human mucins with impact in cancer biology. Crit Rev Clin Lab Sci 41:189–231
Bermbach U, Faulstich H (1990) Epidermal growth factor labeled β-amanitin-poly-L-ornithine: preparation and evidence for specific cytotoxicity. Biochemistry 29:6839–6845
Brockhausen I (1999) Pathways of O-glycan biosynthesis in cancer cells. Biochim Biophys Acta 1473:67–95
Brossart P, Schneider A, Dill P, Schammann T, Grünebach F, Wirths S, Kanz L, Bühring H-J, Brugger W (2001) The epithelial tumor antigen MUC1 is expressed in haematological malignancies and is recognized by MUC1-specific cytotoxic T-lymphocytes. Cancer Res 61:6846–6850
Brugger W, Bühring H-J, Grünebach F, Vogel W, Kaul S, Müller R, Brümmendorf TH, Ziegler BL, Rappold I, Brossart P, Scheding S, Kanz L (1999) Expression of MUC-1 epitopes on normal bone marrow: implications for the detection of micrometastatic tumor cells. J Clin Oncol 17:1535–1544
Burchell JM, Mungul A, Taylor-Papadimitriou J (2001) O-linked glycosylation in the mammary gland: changes that occur during malignancy. J Mammary Gland Biol Neoplasia 6:355–363
Cao Y, Blohm D, Ghadimi BM, Stosiek P, Xing P-X, Karsten U (1997a) Mucins (MUC1 and MUC3) of gastrointestinal and breast epithelia reveal different and heterologeous tumor-associated aberrations in glycosylation. J Histochem Cytochem 45:1547–1557
Cao Y, Schlag PM, Karsten U (1997b) Immunodetection of epithelial mucin (MUC1, MUC3) and mucin-associated glycotopes (TF, Tn, and sialosyl-Tn) in benign and malignant lesions of colonic epithelium: apolar localization corresponds to malignant transformation. Virchows Arch 431:159–166
Cao Y, Karsten U, Hilgers J (1998) Immunohistochemical characterization of a panel of 56 antibodies with normal human small intestine, colon, and breast tissues. Tumor Biol 19 (suppl 1):88–99
Cao Y, Karsten U (2001) Binding pattern of 51 monoclonal antibodies to peptide and carbohydrate epitopes of the epithelial mucin (MUC1) on tissue sections of adenolymphomas of the parotid (Warthin’s tumours): role of epitope masking by glycans. Histochem Cell Biol 115:349–356
Chang J-F, Zhao H-L, Phillips J, Greenburg G (2000) The epithelial mucin, MUC1, is expressed on resting T lymphocytes and can function as a negative regulator of T cell activation. Cell Immunol 201:83–88
Correa I, Plunkett T, Vlad A, Mungul A, Candelora-Kettel J, Burchell JM, Taylor-Papadimitriou J, Finn OJ (2003) Form and pattern of MUC1 expression on T cells activated in vivo or in vitro suggests a function in T-cell migration. Immunology 108:32–41
Dent GA, Civalier CJ, Brecher ME, Bentley SA (1999) MUC1 expression in hematopoietic tissues. Am J Clin Pathol 111:741–747
Duffy MJ (1999) CA 15-3 and related mucins as circulating markers in breast cancer. Ann Clin Biochem 36:579–586
Fattorossi A, Bataglia A, Malinconico P, Stoler AB, Andreocci L, Parente D, Coscarella A, Maggiano N, Perillo A, Pierelli Luca, Scambia G (2002) Constitutive and inducible expression of the epithelial antigen MUC1 (CD227) in human T Cells. Exp Cell Res 280:107–118
Finn OJ, Jerome KR, Henderson RA, Pecher G, Domenech N, Magarian-Blander J, Barratt-Boyes SM (1995) MUC-1 epithelial tumor mucin-based immunity and cancer vaccines. Immunol Rev 145:61–89
Fontenot JD, Mariappan SVS, Catasti P, Domenech N, Finn OJ, Gupta G (1995) Structure of a tumor associated antigen containing a tandemly repeated immunodominant epitope. J Biomol Struct Dyn 13:245–260
Goletz S, Cao Y, Danielczyk A, Ravn P, Schöber U, Karsten U (2003) Thomsen-Friedenreich antigen: the hidden tumor antigen. Adv Exp Med Biol 535:147–162
Hamanaka Y, Suehiro Y, Fukui M, Shikichi K, Imai K, Hinoda Y (2003) Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int J Cancer 103:97–100
Hayes DF, Zurawski VR, Kufe DW (1986) Comparison of circulating CA15-3 and carcinoembryonic antigen in patients with breast cancer. J Clin Oncol 4:1542–1550
Henderikx P, Coolen-van Neer N, Jacobs A, van der Linden E, Arends JW, Mullberg J, Hoogenboom HR (2002) A human immunoglobulin G1 antibody originating from an in vitro-selected Fab phage antibody binds avidly to tumor-associated MUC1 and is efficiently internalized. Am J Pathol 160:1597–1608
Ho JJ, Chung YS, Yuan M, Henslee JG, Kim YS (1992) Differences in expression of SPan-1 and CA15-3 antigens in blood and tissues. Int J Cancer 52:693–700
Hollingsworth MA, Swanson BJ (2004) Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 4:45–60
Karsten U, Butschak G, Cao Y, Goletz S, Hanisch F-G (1995) A new monoclonal antibody (A78-G/A7) to the Thomsen-Friedenreich pan-tumor antigen. Hybridomas 14:37–44
Karsten U, Diotel C, Klich G, Paulsen H, Goletz S, Müller S, Hanisch F-G (1998) Enhanced binding of antibodies to the DTR motif of MUC1 tandem repeat peptide is mediated by site-specific glycosylation. Cancer Res 58:2541–2549
Karsten U, Serttas N, Paulsen H, Danielczyk A, Goletz S (2004) Binding patterns of DTR-specific antibodies reveal a glycosylation-conditioned tumor-specific epitope of the epithelial mucin (MUC1). Glycobiology 14:681–692
Karsten U, von Mensdorff-Pouilly S, Goletz S (2005) What makes MUC1 a tumor antigen? Tumor Biol 26:217–220
Kwa HB, Wesseling J, Verhoeven AH, van Zandwijk N, Hilkens J (1996) Immunoscintigraphy of small-cell lung cancer xenografts with anti neural cell adhesion molecule monoclonal antibody, 123C3: improvement of tumour uptake by internalisation. Br J Cancer 73:439–446
Leong CF, Raudhawati O, Cheong SK, Sivagengei K, Noor Hamidah H (2003) Epithelial membrane antigen (EMA) or MUC1 expression in monocytes and monoblasts. Pathology 35:422–427
Litvinov SV, Hilkens J (1993) The epithelial sialomucin, episialin, is sialylated during recycling. J Biol Chem 268:21364–21371
Lloyd KO, Burchell J, Kudryashov V, Yin BWT, Taylor-Papadimitriou J (1996) Comparison of o-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. J Biol Chem 271:33325–33334
Nikula TK, Curcio MJ, Brechbiel MW, Gansow OA, Finn RD, Scheinberg DA (1995) A rapid, single vessel method for preparation of clinical grade ligand conjugated monoclonal antibodies. Nucl Med Biol 22:387–390
Pietersz GA, Wenjun L, Krauer K, Baker T, Wreschner D, McKenzie IF (1997) Comparison of the biological properties of two anti-mucin-1 antibodies prepared for imaging and therapy. Cancer Immunol Immunother 44:323–328
Potamianos S, Varvarigou AD, Archimandritis SC (2000) Radioimmunoscintigraphy and radioimmunotherapy in cancer: principles and application. Anticancer Res 20:925–948
Press OW, Shan D, Howell-Clark J, Eary J, Appelbaum FR, Matthews D, King DJ, Haines AM, Hamann P, Hinman L, Shochat D, Bernstein ID (1996) Comparative metabolism and retention of iodine-125, yttrium-90, and indium-111 radioimmunoconjugates by cancer cells. Cancer Res 56:2123–2129
Rye PD, Price MR (1998) ISOBM TD-4 International workshop on monoclonal antibodies against MUC1. Tumor Biol 19(suppl 1):1–152
Safi F, Kohler I, Röttinger E, Berger H-G (1991) The value of the tumor marker CA 15-3 in diagnosing and monitoring breast cancer. Cancer 68:574–582
Schuman J, Koganty RR, Longenecker BM, Campbell AP (2003) Probing the conformational and dynamic effects of O-glycosylation within the immunodominant region of a MUC1 peptide tumor antigen. J Pept Res 61:91–108
Seifart K, Sekris CF (1969) Alpha-amanitin, a specific inhibitor of transcription by mammalian RNA polymerase. Z Naturforsch B34:1538–1542
Snijdewint FGM, von Mensdorff-Pouilly S, Karuntu-Wanamarta AG, Verstraeten AA, Livingston PO, Hilgers J, Kenemans P (2001) Antibody-dependent cell-mediated cytotoxicity can be induced by MUC1 peptide vaccination of breast cancer patients. Int J Cancer 93:97–106
Stieber P, Molina R, Chan DW, Fritsche HA, Beyrau R, Bonfrer JM, Filella X, Gornet TG, Hoff T, Jager W, van Kamp GJ, Nagel D, Peisker K, Sokoll LJ, Troalen F, Untch M, Domke I (2003) Clinical evaluation of the Elecsys CA 15-3 test in breast cancer patients. Clin Lab 49:15–24
Taylor-Papadimitriou J, Burchell J, Miles DW, Dalziel M (1999) MUC1 and Cancer. Biochim Biophys Acta 1455:301–313
Treon SP, Mollick JA, Urashima M, Teoh G, Chauhan D, Ogata A, Raje N, Hilgers JHM, Nadler L, Belch AR, Pilarski LM, Anderson KC (1999) Muc-1 core protein is expressed on multiple myeloma cells and is induced by dexamethasone. Blood 93:1287–1298
Treon SP, Maimonis P, Bua D, Young G, Raje N, Mollick J, Chauhan D, Tai Y-T, Hideshima T, Shima Y, Hilgers J, von Mensdorff-Pouilly S, Belch AR, Pilarski LM, Anderson KC (2000) Elevated soluble MUC1 levels and decreased anti-MUC1 antibody levels in patients with multiple myeloma. Blood 96:3147–3153
Woodward MP, Young WW, Bloodgood RA (1985) Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods 78:143–153
Acknowledgements
We are grateful to Hans Paulsen (Hamburg, Germany) for MUC1-derived peptides, to Heinz Faulstich (Heidelberg, Germany) for the preparation of β-amanitin-antibody conjugates and for providing β-amanitin, and to Silvia von Mensdorff-Pouilly (Amsterdam, the Netherlands) for providing a standard probe for serum MUC1 detection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Danielczyk, A., Stahn, R., Faulstich, D. et al. PankoMab: a potent new generation anti-tumour MUC1 antibody. Cancer Immunol Immunother 55, 1337–1347 (2006). https://doi.org/10.1007/s00262-006-0135-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-006-0135-9