Abstract
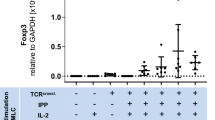
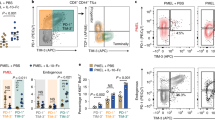
Clinical strategies to exploit Vγ2Vδ2 T cell responses for immunotherapy are confronted with short-term increases in cell levels or activity and the development of anergy that reduces the response to therapy with succeeding treatments. We are exploring strategies to increase the yield and durability of elicited Vγ2Vδ2 T cell responses. One approach focuses on the mammalian target of rapamycin (mTOR), which is important for regulating T cell metabolism and function. In Vγ2Vδ2 T cells, mTOR phosphorylates the S6K1 and eIF4EBP1 signaling intermediates after antigen stimulation. Rapamycin inhibited these phosphorylation events without impacting Akt or Erk activation, even though specific inhibition of Akt or Erk in turn reduced the activation of mTOR. The effects of rapamycin on the T cell receptor signaling pathway lead to increased proliferation of treated and antigen-exposed Vγ2Vδ2 cells. Rapamycin altered the phenotype of antigen-specific Vγ2Vδ2 cells by inducing a population shift from CD62L + CD69− to CD62L-CD69+, higher expression of CD25 or Bcl-2, lower levels of CCR5 and increased resistance to Fas-mediated cellular apoptosis. These changes were consistent with rapamycin promoting cell activation while decreasing the susceptibility to cell death that might occur by CCR5 or Fas signaling. Rapamycin treatment during antigen-stimulation of Vγ2Vδ2 T cells may be a strategy for overcoming current obstacles in tumor immunotherapy.





Similar content being viewed by others
References
Carding SR, Egan PJ (2002) Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol 2(5):336–345. doi:10.1038/nri797
Alexander AA, Maniar A, Cummings JS, Hebbeler AM, Schulze DH, Gastman BR, Pauza CD, Strome SE, Chapoval AI (2008) Isopentenyl pyrophosphate-activated cd56 + gamma}{delta T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res 14(13):4232–4240. doi:10.1158/1078-0432.CCR-07-4912
Qin G, Mao H, Zheng J, Sia SF, Liu Y, Chan PL, Lam KT, Peiris JS, Lau YL, Tu W (2009) Phosphoantigen-expanded human gammadelta T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J Infect Dis 200(6):858–865. doi:10.1086/605413
Bonneville M, Scotet E (2006) Human vgamma9vdelta2 T cells: promising new leads for immunotherapy of infections and tumors. Curr Opin Immunol 18(5):539–546. doi:10.1016/j.coi.2006.07.002
Caccamo N, Meraviglia S, Scarpa F, La Mendola C, Santini D, Bonanno CT, Misiano G, Dieli F, Salerno A (2008) Aminobisphosphonate-activated gammadelta T cells in immunotherapy of cancer: doubts no more. Expert Opin Biol Ther 8(7):875–883. doi:10.1517/14712598.8.7.875
Wang L, Das H, Kamath A, Bukowski JF (2001) Human v gamma 2v delta 2 T cells produce IFN-gamma and TNF-alpha with an on/off/on cycling pattern in response to live bacterial products. J Immunol 167(11):6195–6201
Li H, Luo K, Pauza CD (2008) TNF-alpha is a positive regulatory factor for human vgamma2 vdelta2 T cells. J Immunol 181(10):7131–7137
Viey E, Fromont G, Escudier B, Morel Y, Da Rocha S, Chouaib S, Caignard A (2005) Phosphostim-activated gamma delta t cells kill autologous metastatic renal cell carcinoma. J Immunol 174(3):1338–1347
Liu Z, Guo BL, Gehrs BC, Nan L, Lopez RD (2005) Ex vivo expanded human vgamma9vdelta2 + gammadelta-T cells mediate innate antitumor activity against human prostate cancer cells in vitro. J Urol 173(5):1552–1556
Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR (1995) Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature 375(6527):155–158. doi:10.1038/375155a0
Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M (2000) Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 96(2):384–392
Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP (2003) Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood 102(1):200–206. doi:10.1182/blood-2002-12-3665
Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, Arcara C, Valerio MR, Meraviglia S, Di Sano C, Sireci G, Salerno A (2003) Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood 102(6):2310–2311. doi:10.1182/blood-2003-05-1655
Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D’Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC (2007) Targeting human gamma}delta T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res 67(15):7450–7457. doi:10.1158/0008-5472.CAN-07-0199
Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, Minato N, Toma H (2007) Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother 56(4):469–476. doi:10.1007/s00262-006-0199-6
Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I, Galea C, Salot S, Saiagh S, Audrain M, Rimbert M, Lafaye-de Micheaux S, Tiollier J, Negrier S (2008) Phase-i study of innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 57(11):1599–1609. doi:10.1007/s00262-008-0491-8
Sicard H, Ingoure S, Luciani B, Serraz C, Fournie JJ, Bonneville M, Tiollier J, Romagne F (2005) In vivo immunomanipulation of v gamma 9v delta 2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol 175(8):5471–5480
Vezina C, Kudelski A, Sehgal SN (1975) Rapamycin (ay-22, 989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 28(10):721–726
Saunders RN, Metcalfe MS, Nicholson ML (2001) Rapamycin in transplantation: a review of the evidence. Kidney Int 59(1):3–16. doi:10.1046/j.1523-1755.2001.00460.x
Dittrich E, Schmaldienst S, Soleiman A, Horl WH, Pohanka E (2004) Rapamycin-associated post-transplantation glomerulonephritis and its remission after reintroduction of calcineurin-inhibitor therapy. Transpl Int 17(4):215–220. doi:10.1007/s00147-004-0700-0
Izzedine H, Brocheriou I, Frances C (2005) Post-transplantation proteinuria and sirolimus. N Engl J Med 353(19):2088–2089. doi:10.1056/NEJM200511103531922
Singer SJ, Tiernan R, Sullivan EJ (2000) Interstitial pneumonitis associated with sirolimus therapy in renal-transplant recipients. N Engl J Med 343(24):1815–1816
Thaunat O, Beaumont C, Chatenoud L, Lechaton S, Mamzer-Bruneel MF, Varet B, Kreis H, Morelon E (2005) Anemia after late introduction of sirolimus may correlate with biochemical evidence of a chronic inflammatory state. Transplantation 80(9):1212–1219
Thomson AW, Turnquist HR, Raimondi G (2009) Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 9(5):324–337. doi:10.1038/nri2546
Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM (2006) Ablation in mice of the mTORc components raptor, rictor, or mlst8 reveals that mTORc2 is required for signaling to Akt-foxo and pkcalpha, but not s6k1. Dev Cell 11(6):859–871. doi:10.1016/j.devcel.2006.10.007
Weichhart T, Saemann MD (2009) The multiple facets of mTOR in immunity. Trends Immunol 30(5):218–226. doi:10.1016/j.it.2009.02.002
Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B (2008) Toll-like receptor-mediated induction of type i interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive pi(3)k-mTOR-p70s6k pathway. Nat Immunol 9(10):1157–1164. doi:10.1038/ni.1645
Turnquist HR, Cardinal J, Macedo C, Rosborough BR, Sumpter TL, Geller DA, Metes D, Thomson AW (2010) mTOR and GSK-3 shape the CD4+ T cell stimulatory and differentiation capacity of myeloid dc following exposure to LPS. Blood. doi:10.1182/blood-2009-10-251488
Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA (2008) Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol 9(5):513–521. doi:10.1038/ni.1603
Haxhinasto S, Mathis D, Benoist C (2008) The Akt-mTOR axis regulates de novo differentiation of CD4+ FOXp3+ cells. J Exp Med 205(3):565–574. doi:10.1084/jem.20071477
Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R (2009) Mtor regulates memory CD8 T cell differentiation. Nature 460(7251):108–112. doi:10.1038/nature08155
Gilliam BL, Heredia A, Devico A, Le N, Bamba D, Bryant JL, Pauza CD, Redfield RR (2007) Rapamycin reduces ccr5 mRNA levels in macaques: potential applications in HIV-1 prevention and treatment. AIDS 21(15):2108–2110. doi:10.1097/QAD.0b013e3282f02a4f
Papadopoulos NG, Dedoussis GV, Spanakos G, Gritzapis AD, Baxevanis CN, Papamichail M (1994) An improved fluorescence assay for the determination of lymphocyte-mediated cytotoxicity using flow cytometry. J Immunol Methods 177(1–2):101–111
Li H, Pauza CD (2009) Effects of 15-deoxy-delta12, 14-prostaglandin j2 (15d-pgj2) and rosiglitazone on human gammadelta2 t cells. PLoS One 4(11):e7726. doi:10.1371/journal.pone.0007726
Murooka TT, Wong MM, Rahbar R, Majchrzak-Kita B, Proudfoot AE, Fish EN (2006) Ccl5-ccr5-mediated apoptosis in t cells: requirement for glycosaminoglycan binding and ccl5 aggregation. J Biol Chem 281(35):25184–25194. doi:10.1074/jbc.M603912200
Tikhonov I, Deetz CO, Paca R, Berg S, Lukyanenko V, Lim JK, Pauza CD (2006) Human vgamma2vdelta2 T cells contain cytoplasmic rantes. Int Immunol 18(8):1243–1251. doi:10.1093/intimm/dxl055
Cory S, Huang DC, Adams JM (2003) The bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22(53):8590–8607. doi:10.1038/sj.onc.1207102
Brandes M, Willimann K, Moser B (2005) Professional antigen-presentation function by human gammadelta T cells. Science 309(5732):264–268. doi:10.1126/science.1110267
Strauss L, Czystowska M, Szajnik M, Mandapathil M, Whiteside TL (2009) Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS One 4 (6):e5994. doi:10.1371/journal.pone.0005994
Schall TJ, Bacon K, Toy KJ, Goeddel DV (1990) Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine rantes. Nature 347(6294):669–671. doi:10.1038/347669a0
Mellado M, de Ana AM, Moreno MC, Martinez C, Rodriguez-Frade JM (2001) A potential immune escape mechanism by melanoma cells through the activation of chemokine-induced T cell death. Curr Biol 11(9):691–696
Cartier L, Dubois-Dauphin M, Hartley O, Irminger-Finger I, Krause KH (2003) Chemokine-induced cell death in ccr5-expressing neuroblastoma cells. J Neuroimmunol 145(1–2):27–39
Algeciras-Schimnich A, Vlahakis SR, Villasis-Keever A, Gomez T, Heppelmann CJ, Bou G, Paya CV (2002) Ccr5 mediates fas- and caspase-8 dependent apoptosis of both uninfected and HIV infected primary human CD4 T cells. AIDS 16(11):1467–1478
Calastretti A, Rancati F, Ceriani MC, Asnaghi L, Canti G, Nicolin A (2001) Rapamycin increases the cellular concentration of the bcl-2 protein and exerts an anti-apoptotic effect. Eur J Cancer 37(16):2121–2128
Cummings JS, Cairo C, Armstrong C, Davis CE, Pauza CD (2008) Impacts of HIV infection on vgamma2vdelta2 T cell phenotype and function: a mechanism for reduced tumor immunity in aids. J Leukoc Biol 84(2):371–379. doi:10.1189/jlb.1207847
Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW (2007) Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol 178(11):7018–7031
Acknowledgments
We would like to thank Cheryl Armstrong for technical assistance. We are grateful to Maria S Salvato for critical reading of the manuscript. This work was supported by PHS grant CA142458 (C.D.P.).
Conflict of interest
The authors have no financial conflicts of interest related to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Pauza, C.D. Rapamycin increases the yield and effector function of human γδ T cells stimulated in vitro. Cancer Immunol Immunother 60, 361–370 (2011). https://doi.org/10.1007/s00262-010-0945-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-010-0945-7