Abstract
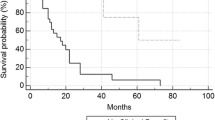
Few immunotherapy compounds have demonstrated a direct link between the predicted mode of action of the product and benefit to the patient. Since cancer vaccines are thought to have a delayed therapeutic effect, identification of the active moiety may enable the development of an early marker of efficacy. Patients with renal cancer and requiring first-line treatment for metastatic disease were randomized 1:1 to receive MVA-5T4 (TroVax®) or placebo alongside Sunitinib, IL-2 or IFN-α in a multicentre phase III trial. Antibody responses were quantified following the 3rd and 4th vaccinations. A surrogate for 5T4 antibody response (the immune response surrogate; IRS) was constructed and then used in a survival analysis to evaluate treatment benefit. Seven hundred and thirty-three patients were randomized, and immune responses were assessed in 590 patients. A high 5T4 antibody response was associated with longer survival within the MVA–5T4-treated group. The IRS was constructed as a linear combination of pre-treatment 5T4 antibody levels, hemoglobin and hematocrit and was shown to be a significant predictor of treatment benefit in the phase III study. Importantly, the IRS was also associated with antibody response and survival in an independent dataset comprising renal, colorectal and prostate cancer patients treated with MVA–5T4 in phase I–II studies. The derivation of the IRS formed part of an exploratory, retrospective analysis; however, if confirmed in future studies, the results have important implications for the development and use of the MVA–5T4 vaccine and potentially for other similar vaccines.


Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics, 2007. CA Cancer J Clin 57(1):43–66
Costa LJ, Drabkin HA (2007) Renal cell carcinoma: new developments in molecular biology and potential for targeted therapies. Oncologist 12(12):1404–1415
Hole N, Stern PL (1988) A 72 kD trophoblast glycoprotein defined by a monoclonal antibody. Br J Cancer 57(3):239–246
Southall PJ, Boxer GM, Bagshawe KD, Hole N, Bromley M, Stern PL (1990) Immunohistological distribution of 5T4 antigen in normal and malignant tissues. Br J Cancer 61(1):89–95
Starzynska T, Marsh PJ, Schofield PF, Roberts SA, Myers KA, Stern PL (1994) Prognostic significance of 5T4 oncofetal antigen expression in colorectal carcinoma. Br J Cancer 69(5):899–902
Harrop R, Shingler W, Kelleher M, De Belin J, Treasure P (2010) Cross-trial analysis of immunological and clinical data resulting from phase I and II trials of MVA-5T4 (TroVax®) in colorectal, renal and prostate cancer patients. J Immunother (in press)
Amato RJ, Drury N, Naylor S, Jac J, Saxena S, Cao A, Hernandez-McClain J, Harrop R (2008) Vaccination of prostate cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax): a phase 2 trial. J Immunother 31(6):577–585
Amato RJ, Shingler W, Goonewardena M, de Belin J, Naylor S, Jac J, Willis J, Saxena S, Hernandez-McClain J, Harrop R (2009) Vaccination of renal cell cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) alone or administered in combination with interferon-alpha (IFN-alpha): a phase 2 trial. J Immunother 32(7):765–772
Amato RJ, Shingler W, Naylor S, Jac J, Willis J, Saxena S, Hernandez-McClain J, Harrop R (2008) Vaccination of renal cell cancer patients with modified vaccinia Ankara delivering tumor antigen 5T4 (TroVax) administered with interleukin 2: a phase II trial. Clin Cancer Res 14(22):7504–7510
Elkord E, Dangoor A, Drury NL, Harrop R, Burt DJ, Drijfhout JW, Hamer C, Andrews D, Naylor S, Sherlock D, Hawkins RE, Stern PL (2008) An MVA-based vaccine targeting the oncofetal antigen 5T4 in patients undergoing surgical resection of colorectal cancer liver metastases. J Immunother 31(9):820–829
Harrop R, Connolly N, Redchenko I, Valle J, Saunders M, Ryan MG, Myers KA, Drury N, Kingsman SM, Hawkins RE, Carroll MW (2006) Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin Cancer Res 12(11 Pt 1):3416–3424
Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW, Kingsman SM, Naylor S, Melcher A, Nicholls J, Wassan H, Habib N, Anthoney A (2007) Vaccination of colorectal cancer patients with modified vaccinia Ankara encoding the tumor antigen 5T4 (TroVax) given alongside chemotherapy induces potent immune responses. Clin Cancer Res 13:4487–4494
Kaufman HL, Taback B, Sherman W, Kim DW, Shingler WH, Moroziewicz D, DeRaffele G, Mitcham J, Carroll MW, Harrop R, Naylor S, Kim-Schulze S (2009) Phase II trial of Modified Vaccinia Ankara (MVA) virus expressing 5T4 and high dose Interleukin-2 (IL-2) in patients with metastatic renal cell carcinoma. J Transl Med 7:2
Amato RJ, Hawkins RE, Kaufman HL, Thompson JA, Tomczak P, Szczylik C, McDonald M, Eastty S, Shingler WH, de Belin J, Goonewardena M, Naylor S, Harrop R (2010) Vaccination of metastatic renal cancer patients with MVA-5T4: a randomized, double blind, placebo controlled phase III study. Clin Cancer Res. doi:10.1158/1078-0432.ccr-10-2082. (in press)
Mire-Sluis AR, Barrett YC, Devanarayan V, Koren E, Liu H, Maia M, Parish T, Scott G, Shankar G, Shores E, Swanson SJ, Taniguchi G, Wierda D, Zuckerman LA (2004) Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J Immunol Methods 289(1–2):1–16
Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW, Kingsman SM, Naylor S, Griffiths R, Steven N, Hawkins RE (2008) Vaccination of colorectal cancer patients with TroVax given alongside chemotherapy (5-fluorouracil, leukovorin and irinotecan) is safe and induces potent immune responses. Cancer Immunol Immunother 57(7):977–986
Hawkins RE, Macdermott C, Shablak A, Hamer C, Thistlethwaite F, Drury NL, Chikoti P, Shingler W, Naylor S, Harrop R (2009) Vaccination of patients with metastatic renal cancer with modified vaccinia Ankara encoding the tumor antigen 5T4 (TroVax) given alongside interferon-alpha. J Immunother 32(4):424–429
Dallman PR (1987) Iron deficiency and the immune response. Am J Clin Nutr 46(2):329–334
Ekiz C, Agaoglu L, Karakas Z, Gurel N, Yalcin I (2005) The effect of iron deficiency anemia on the function of the immune system. Hematol J 5(7):579–583
Zhu J, Martinez J, Huang X, Yang Y (2007) Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood 109(2):619–625
Hutchens MA, Luker KE, Sonstein J, Nunez G, Curtis JL, Luker GD (2008) Protective effect of toll-like receptor 4 in pulmonary vaccinia infection. PLoS Pathog 4(9):e1000153
Lin T, Kwak YH, Sammy F, He P, Thundivalappil S, Sun G, Chao W, Warren HS (2010) Synergistic inflammation is induced by blood degradation products with microbial toll-like receptor agonists and is blocked by hemopexin. J Infect Dis 202(4):624–632
Berry DA (2010) The hazards of endpoints. J Natl Cancer Inst 102(18):1376–1377
Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, Humphrey R, Blumenstein B, Old L, Wolchok J (2010) Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 102(18):1388–1397
Acknowledgment
This paper is dedicated to the memory of Dr. Sue Kingsman who gave so much of her life to the discovery and development of novel treatments for areas of unmet medical need.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harrop, R., Shingler, W.H., McDonald, M. et al. MVA–5T4-induced immune responses are an early marker of efficacy in renal cancer patients. Cancer Immunol Immunother 60, 829–837 (2011). https://doi.org/10.1007/s00262-011-0993-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-0993-7