Abstract
Introduction
Novel breast cancer risk-reducing strategies for individuals with germline mutations of the BRCA1 and/or BRCA2 genes are urgently needed. Identification of antigenic targets that are expressed in early cancers, but absent in normal breast epithelium of these high-risk individuals, could provide the basis for the development of effective immunoprophylactic strategies. Cancer testis (CT) antigens are potential candidates because their expression is restricted to tumors, and accumulating data suggest that they play important roles in cellular proliferation, stem cell function, and carcinogenesis. The objective of this study was to examine the expression of CT antigens and their frequency in BRCA-associated breast cancers.
Methods
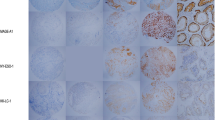
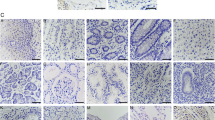
Archived breast cancer tissues (n = 26) as well as morphologically normal breast tissues (n = 7) from women carrying deleterious BRCA 1 and/or 2 mutations were obtained for antigen expression analysis by immunohistochemistry. Expression of the following CT antigens was examined: MAGE-A1, MAGE-A3, MAGE-A4, MAGE-C1.CT7, NY-ESO-1, MAGE-C2/CT10, and GAGE.
Results
CT antigens were expressed in 16/26 (61.5%, 95% CI 43–80%) of BRCA-associated cancers, including in situ tumors. Thirteen of twenty-six (50%) breast cancers expressed two or more CT antigens; three cancers expressed all seven CT antigens. MAGE-A was expressed in 13/26 (50%) of cancers, NY-ESO-1 was expressed in 10/26 (38%) of tumors. In contrast, none of the CT antigens were expressed in adjacent or contralateral normal breast epithelium (P = 0.003).
Conclusions
We report a high CT antigen expression rate in BRCA-associated breast cancer as well as the lack of expression of these antigens in benign breast tissue of carriers, identifying CT antigens as potential vaccine targets for breast cancer prevention in these high-risk individuals.



Similar content being viewed by others
References
Chen S, Parmigiani G (2007) Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 25(11):1329–1333
Frost MH et al (2000) Long-term satisfaction and psychological and social function following bilateral prophylactic mastectomy. JAMA 284(3):319–324
van Oostrom I et al (2003) Long-term psychological impact of carrying a BRCA1/2 mutation and prophylactic surgery: a 5-year follow-up study. J Clin Oncol 21(20):3867–3874
Michelsen TM, Dorum A, Dahl AA (2009) A controlled study of mental distress and somatic complaints after risk-reducing salpingo-oophorectomy in women at risk for hereditary breast ovarian cancer. Gynecol Oncol 113(1):128–133
Kurian AW, Sigal BM, Plevritis SK (2010) Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol 28(2):222–231
Visvanathan K et al (2009) American society of clinical oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol 27(19):3235–3258
Finn OJ (2008) Cancer immunology. N Engl J Med 358(25):2704–2715
Disis ML (2010) The ultimate in cancer chemoprevention: cancer vaccines. Cancer Prev Res (Phila) 3(4):406–409
Lollini PL et al (2006) Vaccines for tumour prevention. Nat Rev Cancer 6(3):204–216
Simpson AJ et al (2005) Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 5(8):615–625
Valmori D et al (2007) Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci USA 104(21):8947–8952
Atanackovic D et al (2008) Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc Natl Acad Sci USA 105(5):1650–1655
Jungbluth AA et al (2005) The cancer-testis antigens CT7 (MAGE-C1) and MAGE-A3/6 are commonly expressed in multiple myeloma and correlate with plasma-cell proliferation. Blood 106(1):167–174
Cronwright G et al (2005) Cancer/testis antigen expression in human mesenchymal stem cells: down-regulation of SSX impairs cell migration and matrix metalloproteinase 2 expression. Cancer Res 65(6):2207–2215
Akers SN, Odunsi K, Karpf AR (2010) Regulation of cancer germline antigen gene expression: implications for cancer immunotherapy. Future Oncol 6(5):717–732
Yawata T et al (2010) Enhanced expression of cancer testis antigen genes in glioma stem cells. Mol Carcinog 49(6):532–544
Gedye C et al (2009) Cancer/testis antigens can be immunological targets in clonogenic CD133 + melanoma cells. Cancer Immunol Immunother 58(10):1635–1646
Ajiro M et al (2009) Involvement of RQCD1 overexpression, a novel cancer-testis antigen, in the Akt pathway in breast cancer cells. Int J Oncol 35(4):673–681
Cheever MA et al (2009) The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 15(17):5323–5337
Sugita Y et al (2004) NY-ESO-1 expression and immunogenicity in malignant and benign breast tumors. Cancer Res 64(6):2199–2204
Taylor M et al (2007) Breast cancer is a promising target for vaccination using cancer-testis antigens known to elicit immune responses. Breast Cancer Res 9(4):R46
Mischo A et al (2006) Prospective study on the expression of cancer testis genes and antibody responses in 100 consecutive patients with primary breast cancer. Int J Cancer 118(3):696–703
Jungbluth AA et al (2002) CT7 (MAGE-C1) antigen expression in normal and neoplastic tissues. Int J Cancer 99(6):839–845
Jungbluth AA et al (2001) Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer 92(6):856–860
Jungbluth AA et al (2000) Expression of MAGE-antigens in normal tissues and cancer. Int J Cancer 85(4):460–465
Theurillat JP et al (2007) NY-ESO-1 protein expression in primary breast carcinoma and metastases: correlation with CD8 + T-cell and CD79a + plasmacytic/B-cell infiltration. Int J Cancer 120(11):2411–2417
Otte M et al (2001) MAGE-A gene expression pattern in primary breast cancer. Cancer Res 61(18):6682–6687
Grigoriadis A et al (2009) CT-X antigen expression in human breast cancer. Proc Natl Acad Sci USA 106(32):13493–13498
Kavalar R et al (2001) Expression of MAGE tumour-associated antigens is inversely correlated with tumour differentiation in invasive ductal breast cancers: an immunohistochemical study. Virchows Arch 439(2):127–131
Curigliano G et al (2011) Cancer-testis antigen expression in triple-negative breast cancer. Ann Oncol 22(1):98–103
Anders CK, Carey LA (2009) Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer 9 (Suppl 2):S73–S81
Aiyar SE et al (2007) Regulation of clustered gene expression by cofactor of BRCA1 (COBRA1) in breast cancer cells. Oncogene 26(18):2543–2553
Jazaeri AA et al (2004) BRCA1-mediated repression of select X chromosome genes. J Transl Med 2(1):32
Jungbluth AA et al (2000) Monoclonal antibody MA454 reveals a heterogeneous expression pattern of MAGE-1 antigen in formalin-fixed paraffin embedded lung tumours. Br J Cancer 83(4):493–497
Zhuang R et al (2006) Generation of monoclonal antibodies to cancer/testis (CT) antigen CT10/MAGE-C2. Cancer Immun 6:7
Kocher T et al (1995) Identification and intracellular location of MAGE-3 gene product. Cancer Res 55(11):2236–2239
Robbins PF et al (2011) Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol
Dhodapkar MV et al (2003) Expression of cancer/testis (CT) antigens MAGE-A1, MAGE-A3, MAGE-A4, CT-7, and NY-ESO-1 in malignant gammopathies is heterogeneous and correlates with site, stage and risk status of disease. Cancer Immun 3:9
Rimoldi D et al (2000) Anti-MAGE-3 antibody 57B and anti-MAGE-1 antibody 6C1 can be used to study different proteins of the MAGE-A family. Int J Cancer 86(5):749–751
Landry C et al (2000) Monoclonal antibody 57B stains tumor tissues that express gene MAGE-A4. Int J Cancer 86(6):835–841
De Plaen E et al (1994) Structure, chromosomal localization, and expression of 12 genes of the MAGE family. Immunogenetics 40(5):360–369
Hudolin T et al (2006) Immunohistochemical expression of tumor antigens MAGE-A1, MAGE-A3/4, and NY-ESO-1 in cancerous and benign prostatic tissue. Prostate 66(1):13–18
Velazquez EF et al (2007) Expression of the cancer/testis antigen NY-ESO-1 in primary and metastatic malignant melanoma (MM)–correlation with prognostic factors. Cancer Immun 7:11
Odunsi K et al (2003) NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res 63(18):6076–6083
Brichard VG, Lejeune D (2008) Cancer immunotherapy targeting tumour-specific antigens: towards a new therapy for minimal residual disease. Expert Opin Biol Ther 8(7):951–968
Acknowledgments
The authors wish to thank Nelli Ziguridis at the NYUCI Tissue Bank for specimen acquisition and Dr. Franco Muggia for his critical review of the manuscript. Sylvia Adams was supported in part by NCI K23 CA125205, and the NYU Cancer Institute Core facilities were supported by NIH 5P30CA016087-27.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adams, S., Greeder, L., Reich, E. et al. Expression of cancer testis antigens in human BRCA-associated breast cancers: potential targets for immunoprevention?. Cancer Immunol Immunother 60, 999–1007 (2011). https://doi.org/10.1007/s00262-011-1005-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1005-7