Abstract
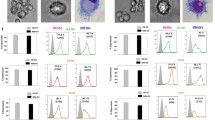
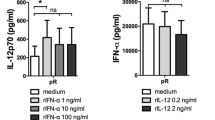
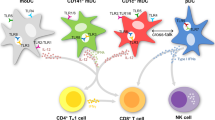
Myeloid-derived suppressor cells (MDSC) are important regulators of the immune system and key players in tumor-induced suppression of T-cell responses. CD14+HLA-DR−/low MDSC have been detected in a great number of malignancies, including melanoma. MDSC are known to be impaired in their ability to differentiate along the myeloid lineage, e.g., into dendritic cells (DC). This is a concern for utilization of monocyte-derived DC for vaccination of patients with melanoma or other cancers exhibiting accumulation of CD14+ MDSC. When producing DC according to standard operating procedures of two currently ongoing clinical trials, we found that MDSC co-purified with monocytes isolated by elutriation. MDSC frequencies did not affect yield or viability of the produced DC, but induced a dose-dependent decrease in DC maturation, ability to take up antigen, migrate and induce T-cell IFNγ production. Changes in DC characteristics were most notable when ‘pathological’ frequencies of >50% CD14+HLA-DR− cells were present in the starting culture. The impaired DC quality could not be explained by altered cytokine production or increased oxidative stress in the cultures. Tracking of HLA-DR− cells throughout the culture period revealed that the observed changes were partially due to the impaired maturation and functionality of the originally HLA-DR− population, but also to their negative effects on HLA-DR+ cells. In conclusion, MDSC could be induced to differentiate into DC but, due to the impairment of overall DC vaccine quality when >50% HLA-DR− cells were present in the starting culture, their removal could be advisable.






Similar content being viewed by others
References
Youn JI, Gabrilovich DI (2010) The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 40(11):2969–2975. doi:10.1002/eji.201040895
Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V (2010) Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol 22(2):238–244. doi:10.1016/j.coi.2010.01.021
Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA, Moldawer LL (2011) A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med 17(3–4):281–292. doi:10.2119/molmed.2010.00178
Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL (2007) MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med 204(6):1463–1474. doi:10.1084/jem.20062602
Greifenberg V, Ribechini E, Rossner S, Lutz MB (2009) Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur J Immunol 39(10):2865–2876. doi:10.1002/eji.200939486
Brimnes MK, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, Svane IM (2010) Increased level of both CD4+FOXP3+ regulatory T cells and CD14+HLA-DR/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol 72(6):540–547. doi:10.1111/j.1365-3083.2010.02463.x
Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L (2007) Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 25(18):2546–2553. doi:10.1200/JCO.2006.08.5829
Gustafson MP, Lin Y, New KC, Bulur PA, O’Neill BP, Gastineau DA, Dietz AB (2010) Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. doi:10.1093/neuonc/noq001
Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F (2008) A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 135(1):234–243. doi:10.1053/j.gastro.2008.03.020
Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB (2011) Immunosuppressive CD14+HLA-DR(low)/− monocytes in B-cell non-Hodgkin lymphoma. Blood 117(3):872–881. doi:10.1182/blood-2010-05-283820
Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI (2006) All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res 66(18):9299–9307. doi:10.1158/0008-5472.CAN-06-1690
Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R (2010) Immature immunosuppressive CD14+HLA-DR−/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res 70(11):4335–4345. doi:10.1158/0008-5472.CAN-09-3767
Schmielau J, Finn OJ (2001) Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Res 61(12):4756–4760
Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, Dietz AB (2010) Immunosuppressive CD14+HLA-DRlow/− monocytes in prostate cancer. Prostate 70(4):443–455. doi:10.1002/pros.21078
Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI (2008) Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med 205(10):2235–2249. doi:10.1084/jem.20080132
Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich D (2003) All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res 63(15):4441–4449
Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, Yancey D, Dahm P, Vieweg J (2008) Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res 14(24):8270–8278. doi:10.1158/1078-0432.CCR-08-0165
Schuler G (2010) Dendritic cells in cancer immunotherapy. Eur J Immunol 40(8):2123–2130. doi:10.1002/eji.201040630
Dietz AB, Padley DJ, Butler GW, Maas ML, Greiner CW, Gastineau DA, Vuk-Pavlovic S (2004) Clinical-grade manufacturing of DC from CD14+ precursors: experience from phase I clinical trials in CML and malignant melanoma. Cytotherapy 6(6):563–570
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363(5):411–422. doi:10.1056/NEJMoa1001294
Suso EM, Dueland S, Rasmussen AM, Vetrhus T, Aamdal S, Kvalheim G, Gaudernack G (2011) hTERT mRNA dendritic cell vaccination: complete response in a pancreatic cancer patient associated with response against several hTERT epitopes. Cancer Immunol Immunother 60(6):809–818. doi:10.1007/s00262-011-0991-9
Aguilera R, Saffie C, Tittarelli A, Gonzalez FE, Ramirez M, Reyes D, Pereda C, Hevia D, Garcia T, Salazar L, Ferreira A, Hermoso M, Mendoza-Naranjo A, Ferrada C, Garrido P, Lopez MN, Salazar-Onfray F (2011) Heat-shock induction of tumor-derived danger signals mediates rapid monocyte differentiation into clinically effective dendritic cells. Clin Cancer Res 17(8):2474–2483. doi:10.1158/1078-0432.CCR-10-2384
Lopez MN, Pereda C, Segal G, Munoz L, Aguilera R, Gonzalez FE, Escobar A, Ginesta A, Reyes D, Gonzalez R, Mendoza-Naranjo A, Larrondo M, Compan A, Ferrada C, Salazar-Onfray F (2009) Prolonged survival of dendritic cell-vaccinated melanoma patients correlates with tumor-specific delayed type IV hypersensitivity response and reduction of tumor growth factor beta-expressing T cells. J Clin Oncol 27(6):945–952. doi:10.1200/JCO.2008.18.0794
Wang S, Hong S, Yang J, Qian J, Zhang X, Shpall E, Kwak LW, Yi Q (2006) Optimizing immunotherapy in multiple myeloma: Restoring the function of patients’ monocyte-derived dendritic cells by inhibiting p38 or activating MEK/ERK MAPK and neutralizing interleukin-6 in progenitor cells. Blood 108(13):4071–4077. doi:10.1182/blood-2006-04-016980
Breloer M, Fleischer B (2008) CD83 regulates lymphocyte maturation, activation and homeostasis. Trends Immunol 29(4):186–194. doi:10.1016/j.it.2008.01.009
Tschoep K, Manning TC, Harlin H, George C, Johnson M, Gajewski TF (2003) Disparate functions of immature and mature human myeloid dendritic cells: implications for dendritic cell-based vaccines. J Leukoc Biol 74(1):69–80
Dieckmann D, Schultz ES, Ring B, Chames P, Held G, Hoogenboom HR, Schuler G (2005) Optimizing the exogenous antigen loading of monocyte-derived dendritic cells. Int Immunol 17(5):621–635. doi:10.1093/intimm/dxh243
de Vries IJ, Lesterhuis WJ, Scharenborg NM, Engelen LP, Ruiter DJ, Gerritsen MJ, Croockewit S, Britten CM, Torensma R, Adema GJ, Figdor CG, Punt CJ (2003) Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res 9(14):5091–5100
Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH (2009) Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 15(6):2148–2157. doi:10.1158/1078-0432.CCR-08-1332
Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I (2006) Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 203(12):2691–2702. doi:10.1084/jem.20061104
Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, Meyer C, Becerra CR, Fishman M, Antonia S, Sporn MB, Liby KT, Rawal B, Lee JH, Gabrilovich DI (2010) Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res 16(6):1812–1823. doi:10.1158/1078-0432.CCR-09-3272
Acknowledgments
This study was supported by grants from the Swedish Cancer Society, the Swedish Medical Research Council, the Cancer Society of Stockholm, the European Union (Grants “EUCAAD” and “DC-THERA”), the Karolinska Institutet, an “ALF-Project” grant from the Stockholm City Council, the Robert Lundgrens Foundation, the Sigurd and Elsa Goljes Memorial foundation and Lars Hiertas Memorial Foundation.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Poschke, I., Mao, Y., Adamson, L. et al. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother 61, 827–838 (2012). https://doi.org/10.1007/s00262-011-1143-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1143-y