Abstract
Purpose
Dendritic cells (DCs) can induce strong tumor-specific T-cell immune responses. Constitutive upregulation of the mitogen-activated protein kinase (MAPK) pathway by a BRAFV600 mutation, which is present in about 50 % of metastatic melanomas, may be linked to compromised function of DCs in the tumor microenvironment. Targeting both MEK and BRAF has shown efficacy in BRAFV600 mutant melanoma.
Methods
We co-cultured monocyte-derived human DCs with melanoma cell lines pretreated with the MEK inhibitor U0126 or the BRAF inhibitor vemurafenib. Cytokine production (IL-12 and TNF-α) and surface marker expression (CD80, CD83, and CD86) in DCs matured with the Toll-like receptor 3/Melanoma Differentiation-Associated protein 5 agonist polyI:C was examined. Additionally, DC function, viability, and T-cell priming capacity were assessed upon direct exposure to U0126 and vemurafenib.
Results
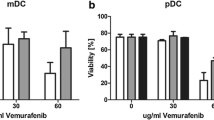
Cytokine production and co-stimulation marker expression were suppressed in polyI:C-matured DCs exposed to melanoma cells in co-cultures. This suppression was reversed by MAPK blockade with U0126 and/or vemurafenib only in melanoma cell lines carrying a BRAFV600E mutation. Furthermore, when testing the effect of U0126 directly on DCs, marked inhibition of function, viability, and DC priming capacity was observed. In contrast, vemurafenib had no effect on DC function across a wide range of dose concentrations.
Conclusions
BRAFV600E mutant melanoma cells modulate DC through the MAPK pathway as its blockade can reverse suppression of DC function. MEK inhibition negatively impacts DC function and viability if applied directly. In contrast, vemurafenib does not have detrimental effects on important functions of DCs and may therefore be a superior candidate for combination immunotherapy approaches in melanoma patients.





Similar content being viewed by others
References
Chapman PB, Hauschild A, Robert C et al (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364:2507–2516. doi:10.1056/NEJMoa1103782
Flaherty KT, Puzanov I, Kim KB et al (2010) Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363:809–819. doi:10.1056/NEJMoa1002011
Sosman JA, Kim KB, Schuchter L et al (2012) Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 366:707–714. doi:10.1056/NEJMoa1112302
Flaherty KT, Infante JR, Daud A et al (2012) Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. doi:10.1056/NEJMoa1210093
Flaherty KT, Robert C, Hersey P et al (2012) Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. doi:10.1056/NEJMoa1203421
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723. doi:10.1056/NEJMoa1003466
Robert C, Thomas L, Bondarenko I et al (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364:2517–2526. doi:10.1056/NEJMoa1104621
Brahmer JR, Tykodi SS, Chow LQ et al (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–2465. doi:10.1056/NEJMoa1200694
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454. doi:10.1056/NEJMoa1200690
Dudley ME, Yang JC, Sherry R et al (2008) Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 26:5233–5239. doi:10.1200/JCO.2008.16.5449
Zou W (2005) Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 5:263–274. doi:10.1038/nrc1586
Zou W (2006) Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 6:295–307
Yang Z–Z, Novak AJ, Stenson MJ, Witzig TE, Ansell SM (2006) Intratumoral CD4+ CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood 107:3639–3646
Viguier M, Lemaitre F, Verola O, Cho M-S, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L (2004) Foxp3 expressing CD4+ CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol (Baltimore, Md: 1950) 173:1444–1453
Dong H, Chen L (2003) B7–H1 pathway and its role in the evasion of tumor immunity. J Mol Med 81:281–287
Freeman GJ, Long AJ, Iwai Y et al (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192:1027–1034
Wang T, Niu G, Kortylewski M et al (2004) Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med 10:48–54
Yu H, Kortylewski M, Pardoll D (2007) Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7:41–51
Boni A, Cogdill AP, Dang P et al (2010) Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 70:5213–5219. doi:10.1158/0008-5472.CAN-10-0118
Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, Kefford RF, Hersey P, Scolyer RA (2012) Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res 18:1386–1394. doi:10.1158/1078-0432.CCR-11-2479
Adams S, O’Neill DW, Bhardwaj N (2005) Recent advances in dendritic cell biology. J Clin Immunol 25:177–188
Lee AW, Truong T, Bickham K, Fonteneau JF, Larsson M, Da Silva I, Somersan S, Thomas EK, Bhardwaj N (2002) A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine 20(Suppl 4):A8–A22
Gorden A, Osman I, Gai W, He D, Huang W, Davidson A, Houghton AN, Busam K, Polsky D (2003) Analysis of BRAF and N-RAS mutations in metastatic melanoma tissues. Cancer Res 63:3955–3957
Sondergaard JN, Nazarian R, Wang Q et al (2010) Differential sensitivity of melanoma cell lines with BRAFV600E mutation to the specific RAF inhibitor PLX4032. J Transl Med 8:39. doi:10.1186/1479-5876-8-39
Smalley KS, Contractor R, Haass NK, Lee JT, Nathanson KL, Medina CA, Flaherty KT, Herlyn M (2007) Ki67 expression levels are a better marker of reduced melanoma growth following MEK inhibitor treatment than phospho-ERK levels. Br J Cancer 96:445–449. doi:10.1038/sj.bjc.6603596
Callahan M (2011) Defining the effects of MAPK pathway inhibition in human immune cells. Perspectives in Melanoma XV. Imedex, New York, NY
Comin-Anduix B, Chodon T, Sazegar H et al (2010) The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clin Cancer Res 16:6040–6048. doi:10.1158/1078-0432.CCR-10-1911
Flaherty KT, Robert C, Hersey P et al (2012) Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 367:107–114. doi:10.1056/NEJMoa1203421
Sumimoto H, Imabayashi F, Iwata T, Kawakami Y (2006) The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med 203:1651–1656. doi:10.1084/jem.20051848
Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma R, Thompson JF, Kefford RF, Hersey P, Scolyer RA (2011) Selective BRAF inhibitors induce marked T cell infiltration into human metastatic melanoma. Clin Cancer Res. doi:10.1158/1078-0432.CCR-11-2479
Vujanovic L, Ballard W, Thorne SH, Vujanovic NL, Butterfield LH (2012) Adenovirus-engineered human dendritic cells induce natural killer cell chemotaxis via CXCL8/IL-8 and CXCL10/IP-10. Oncoimmunology 1:448–457
Jackson AM, Mulcahy LA, Zhu XW, O’Donnell D, Patel PM (2008) Tumour-mediated disruption of dendritic cell function: inhibiting the MEK1/2-p44/42 axis restores IL-12 production and Th1-generation. Int J Cancer 123:623–632. doi:10.1002/ijc.23530
Bogunovic D, Manches O, Godefroy E, Yewdall A, Gallois A, Salazar AM, Marie I, Levy DE, Bhardwaj N (2011) TLR4 engagement during TLR3-induced proinflammatory signaling in dendritic cells promotes IL-10-mediated suppression of antitumor immunity. Cancer Res 71:5467–5476. doi:10.1158/0008-5472.CAN-10-3988
Gopal YN, Deng W, Woodman SE, Komurov K, Ram P, Smith PD, Davies MA (2010) Basal and treatment-induced activation of AKT mediates resistance to cell death by AZD6244 (ARRY-142886) in BRAF-mutant human cutaneous melanoma cells. Cancer Res 70:8736–8747. doi:10.1158/0008-5472.CAN-10-0902
VanBrocklin MW, Verhaegen M, Soengas MS, Holmen SL (2009) Mitogen-activated protein kinase inhibition induces translocation of BMF to promote apoptosis in melanoma. Cancer Res 69:1985–1994. doi:10.1158/0008-5472.CAN-08-3934
Hong DS, Vence LM, Falchook GS et al (2012) Braf(V600) inhibitor Gsk2118436 targeted inhibition of mutant BRAF in cancer patients does not impair overall immune competency. Clin Cancer Res. doi:10.1158/1078-0432.CCR-11-2515
Puig-Kroger A, Relloso M, Fernandez-Capetillo O, Zubiaga A, Silva A, Bernabeu C, Corbi AL (2001) Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood 98:2175–2182
Escors D, Lopes L, Lin R, Hiscott J, Akira S, Davis RJ, Collins MK (2008) Targeting dendritic cell signaling to regulate the response to immunization. Blood 111:3050–3061. doi:10.1182/blood-2007-11-122408
Ardeshna KM, Pizzey AR, Devereux S, Khwaja A (2000) The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood 96:1039–1046
Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C (2001) A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J Immunol 166:3837–3845
Martinez D, Vermeulen M, von Euw E et al (2007) Extracellular acidosis triggers the maturation of human dendritic cells and the production of IL-12. J Immunol 179:1950–1959
Yu Q, Kovacs C, Yue FY, Ostrowski MA (2004) The role of the p38 mitogen-activated protein kinase, extracellular signal-regulated kinase, and phosphoinositide-3-OH kinase signal transduction pathways in CD40 ligand-induced dendritic cell activation and expansion of virus-specific CD8+ T cell memory responses. J Immunol 172:6047–6056
Oberholzer PA, Kee D, Dziunycz P et al (2012) RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol 30:316–321. doi:10.1200/JCO.2011.36.7680
Su F, Viros A, Milagre C et al (2012) RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med 366:207–215. doi:10.1056/NEJMoa1105358
Hong DS, Vence L, Falchook G et al (2012) BRAF(V600) inhibitor GSK2118436 targeted inhibition of mutant BRAF in cancer patients does not impair overall immune competency. Clin Cancer Res 18:2326–2335. doi:10.1158/1078-0432.CCR-11-2515
Acknowledgments
Melanoma Research Alliance (Young Investigator Award to PAO), Cancer Research Institute (NB).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
262_2012_1389_MOESM1_ESM.ppt
Supplemental Figure S1: Cytokine production is variable across different human melanoma cell lines and can be inhibited with MAPK inhibition. BRAFV600E mutant cell lines (M44, GMEL, SK19, 888-mel) and BRAF WT lines (FM29, SK173, SK197) were cultured in 96-well plates at 250,000 cells/ml, and supernatants were harvested after 48 h. IL-6, IL-8, and IL-10 were measured using CBA (a-c). Mean cytokine levels are shown from at least 5 independent experiments. M44, SK19, 888-mel, and FM29 were cultured for 48 h with the inactive analogue U0124 versus addition of the MEK inhibitor U0126; IL-8 production was measured (d). Bars indicate the percentage of IL-8 production compared to control (U0124), where control is 100 %. Apoptosis was assessed by staining for Annexin V–FITC and 7-AAD in GMEL, 888-mel (both BRAFV600E mutant), FM29, and SK197 cells (both BRAF WT) treated in duplicates with U0126, vemurafenib, U0124, and DMSO (e). Vemu: vemurafenib. (PPT 236 kb)
262_2012_1389_MOESM2_ESM.pptx
Supplemental Figure S2: Suppressed cytokine production of poly:IC-matured DCs co-cultured with melanoma cell lines is not restored by MEK or BRAF inhibition in BRAF WT melanoma cell lines. Melanoma cell line SK173 (BRAF WT) was treated with the MEK inhibitor U0126 or the inactive analogue U0124 and co-cultured with DCs as described in Fig. 1 (a, b). Melanoma cell line SK197 (BRAF WT) was treated with the BRAF inhibitor vemurafenib versus vehicle control (DMSO) and co-cultured with DCs as described in Fig. 1 (c, d). Using CBA, supernatants of the melanoma/DC co-cultures were tested for the production of TNF-α (a, c) and IL-12 (b, d). DCs from 11 donors were analyzed in triplicate in 5 independent experiments (a, b); DCs from 7 donors were analyzed in triplicate in 3 independent experiments (c, d). Mel: melanoma cells; vemu: vemurafenib; NS: not significant. (PPTX 210 kb)
262_2012_1389_MOESM3_ESM.ppt
Supplemental Figure S3: Cell viability of polyI:C-matured DCs is altered by MEK, but not BRAF inhibition. Apoptosis was assessed by staining for Annexin V–FITC and 7-AAD in DCs treated in duplicates with U0126 (a) or vemurafenib (b) at different concentrations prior to polyI:C stimulation. Viability of DCs treated with U0126 or vemurafenib prior to polyI:C stimulation was examined using an ATP-based assay (c). One representative donor out of 6 tested in 3 independent experiments is shown. Bars indicate SD of triplicates. NT: not treated. (PPT 161 kb)
Rights and permissions
About this article
Cite this article
Ott, P.A., Henry, T., Baranda, S.J. et al. Inhibition of both BRAF and MEK in BRAFV600E mutant melanoma restores compromised dendritic cell (DC) function while having differential direct effects on DC properties. Cancer Immunol Immunother 62, 811–822 (2013). https://doi.org/10.1007/s00262-012-1389-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-012-1389-z