Abstract
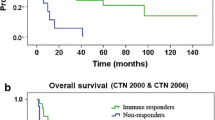
Myeloid-derived suppressor cells (MDSC) are one of the major factors limiting the efficacy of immune therapy. In a clinical trial of patients with extensive stage small cell lung cancer (SCLC), we tested the possibility that targeting MDSC can improve the induction of immune responses by a cancer vaccine. Forty-one patients with extensive stage SCLC were randomized into three arms: arm A—control, arm B—vaccination with dendritic cells transduced with wild-type p53, and arm C—vaccination in combination with MDSC targeted therapy with all-trans-retinoic acid (ATRA). Interim results of the ongoing clinical trial are presented. Pre-treatment levels of MDSC populations in patients from all three arms were similar. Vaccine alone did not affect the proportion of MDSC, whereas in patients treated with ATRA, the MDSC decreased more than twofold (p = 0.02). Before the start of treatment, no patients had detectable p53-specific responses in IFN-γ ELISPOT. Sequential measurements did not show positive p53 responses in any of the 14 patients from arm A. After immunization, only 3 out of 15 patients (20 %) from arm B developed a p53-specific response (p = 0.22). In contrast, in arm C, 5 out of 12 patients (41.7 %) had detectable p53 responses (p = 0.012). The proportion of granzyme B-positive CD8+ T cells was increased only in patients from arm C but not in arm B. Depletion of MDSC substantially improved the immune response to vaccination, suggesting that this approach can be used to enhance the effect of immune interventions in cancer.




Similar content being viewed by others
References
Mellman I, Coukos G, Dranoff G (2011) Cancer immunotherapy comes of age. Nature 480:480–489
Restifo NP, Dudley ME, Rosenberg SA (2012) Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 12:269–281
Pardoll D, Drake C (2012) Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med 209:201–209
Rabinovich GA, Gabrilovich D, Sotomayor EM (2007) Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 25:267–296
Zou W, Chen L (2008) Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 8:467–477
Munn DH, Mellor AL (2007) Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest 117:1147–1154
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V (2012) Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 12:253–268
Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V (2010) Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol 22:238–244
Ostrand-Rosenberg S, Sinha P (2009) Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 182:4499–4506
Filipazzi P, Valenti R, Huber V et al (2007) Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 25:2546–2553
Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R (2010) Immature immunosuppressive CD14 + HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res 70:4335–4345
Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ (2009) Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 58:49–59
Montero AJ, Diaz-Montero CM, Deutsch YE et al (2012) Phase 2 study of neoadjuvant treatment with NOV-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with HER-2 negative clinical stage II-IIIc breast cancer. Breast Cancer Res Treat 132:215–223
Yuan XK, Zhao XK, Xia YC, Zhu X, Xiao P (2011) Increased circulating immunosuppressive CD14(+)HLA-DR(−low) cells correlate with clinical cancer stage and pathological grade in patients with bladder carcinoma. J Int Med Res 39:1381–1391
Eruslanov E, Neuberger M, Daurkin I et al (2012) Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int J Cancer 130:1109–1119
Goedegebuure P, Mitchem JB, Porembka MR, Tan MC, Belt BA, Wang-Gillam A, Gillanders WE, Hawkins WG, Linehan DC (2011) Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer. Curr Cancer Drug Targets 11:734–751
Walter S, Weinschenk T, Stenzl A et al (2012) Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 18:1254–1265
Nikitina EY, Clark JI, van Beynen J, Chada S, Virmani AK, Carbone DP, Gabrilovich DI (2001) Dendritic cells transduced with full-length wild-type p53 generate antitumor cytotoxic T lymphocytes from peripheral blood of cancer patients. Clin Cancer Res 7:127–135
Antonia SJ, Mirza N, Fricke I et al (2006) Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res 12:878–887
Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich DI (2003) All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res 63:4441–4449
Gabrilovich DI, Velders M, Sotomayor E, Kast WM (2001) Mechanism of immune dysfunction in cancer mediated by immature Gr-1 + myeloid cells. J. Immunol 166:5398–5406
Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, Yancey D, Dahm P, Vieweg J (2008) Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res 14:8270–8278
Weiss T, Vitacolonna M, Zoller M (2009) The efficacy of an IL-1alpha vaccine depends on IL-1RI availability and concomitant myeloid-derived suppressor cell reduction. J Immunother 32:552–564
Lee JM, Seo JH, Kim YJ, Kim YS, Ko HJ, Kang CY (2012) The restoration of myeloid-derived suppressor cells as functional antigen-presenting cells by NKT cell help and all-trans-retinoic acid treatment. Int J Cancer 131:741–751
Song X, Guo W, Cui J, Qian X, Yi L, Chang M, Cai Q, Zhao Q (2011) A tritherapy combination of a fusion protein vaccine with immune-modulating doses of sequential chemotherapies in an optimized regimen completely eradicates large tumors in mice. Int J Cancer 128:1129–1138
Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI (2006) All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res 66:9299–9307
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Wieand HS (2005) Randomized phase II trials: what does randomization gain? J Clin Oncol 23:1794–1795
Rocha Lima C, Chiappori A (2003) Treatment of relapsed small-cell lung cancer: a focus on the evolving role of topotecan. Lung Cancer 40:229–236
Nagaraj S, Gabrilovich DI (2010) Myeloid-derived suppressor cells in human cancer. Cancer J (Sudbury Mass) 16:348–353
Nagaraj S, Youn J, Weber H et al (2010) Anti-inflammatory triterpenoid blocks immune suppressive function of myeloid-derived suppressor cells and improves immune response in cancer. Clin Cancer Res 16:1812–1823
Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI (2001) Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol 166:678–689
Solito S, Falisi E, Diaz-Montero CM et al (2011) A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood 118:2254–2265
Gudas LJ, Wagner JA (2011) Retinoids regulate stem cell differentiation. J Cell Physiol 226:322–330
Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI (2007) Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res 67:11021–11028
Gervais A, Leveque J, Bouet-Toussaint F, Burtin F, Lesimple T, Sulpice L, Patard JJ, Genetet N, Catros-Quemener V (2005) Dendritic cells are defective in breast cancer patients: a potential role for polyamine in this immunodeficiency. Breast Cancer Res 7:R326–R335
Mohty M, Morbelli S, Isnardon D, Sainty D, Arnoulet C, Gaugler B, Olive D (2003) All-trans retinoic acid skews monocyte differentiation into interleukin-12-secreting dendritic-like cells. Br J Haematol 122:829–836
Zitvogel L, Kepp O, Kroemer G (2011) Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol 8:151–160
Gabrilovich DI (2007) Combination of chemotherapy and immunotherapy for cancer: a paradigm revisited. The Lancet Oncol 8:2–3
Ramakrishnan R, Gabrilovich DI (2011) Mechanism of synergistic effect of chemotherapy and immunotherapy of cancer. Cancer Immunol Immunother 60:419–423
Dark GG, Calvert AH, Grimshaw R et al (2005) Randomized trial of two intravenous schedules of the topoisomerase I inhibitor liposomal lurtotecan in women with relapsed epithelial ovarian cancer: a trial of the national cancer institute of Canada clinical trials group. J Clin Oncol 23:1859–1866
Trellakis S, Bruderek K, Hütte J, Elian M, Hoffmann TK, Lang S, Brandau S (2012) Granulocytic myeloid-derived suppressor cells are cryosensitive and their frequency does not correlate with serum concentrations of colony-stimulating factors in head and neck cancer. Innate Immun. http://ini.sagepub.com/content/early/2012/11/16/1753425912463618.full.pdf+html
Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL (2012) Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J Immunol Methods 381:14–22
Golovina TN, Mikheeva T, Brusko TM, Blazar BR, Bluestone JA, Riley JL (2011) Retinoic acid and rapamycin differentially affect and synergistically promote the ex vivo expansion of natural human T regulatory cells. PLoS ONE 6:e15868
Van YH, Lee WH, Ortiz S, Lee MH, Qin HJ, Liu CP (2009) All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-gamma-producing T-cells without affecting Th17 cells. Diabetes 58:146–155
Kwok SK, Park MK, Cho ML, Oh HJ, Park EM, Lee DG, Lee J, Kim HY, Park SH (2012) Retinoic acid attenuates rheumatoid inflammation in mice. J Immunol 189:1062–1071
Arrieta O, Gonzalez-De la Rosa CH, Arechaga-Ocampo E et al (2010) Randomized phase II trial of All-trans-retinoic acid with chemotherapy based on paclitaxel and cisplatin as first-line treatment in patients with advanced non-small-cell lung cancer. J Clin Oncol 28:3463–3471
Acknowledgments
This work was supported by NIH SPORE grant P50 CA119997 to Scott Antonia and Dmitry Gabrilovich and partially supported by Cell Therapy Core at H. Lee Moffitt Cancer Center. We thank Dr. Palucka (Baylor Institute of Immunology) for help with protocol evaluating intracellular cytokine production by T cells.
Conflict of interests
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors Cristina Iclozan, Scott Antonia, and Alberto Chiappori contributed equally to this work.
Rights and permissions
About this article
Cite this article
Iclozan, C., Antonia, S., Chiappori, A. et al. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother 62, 909–918 (2013). https://doi.org/10.1007/s00262-013-1396-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-013-1396-8