Abstract
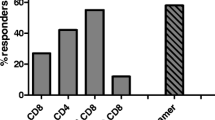
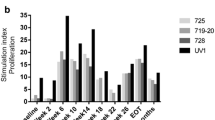
The primary end point of this study was to determine the safety and feasibility of intraprostatic administration of PSA-TRICOM vaccine [encoding transgenes for prostate-specific antigen (PSA) and 3 costimulatory molecules] in patients with locally recurrent or progressive prostate cancer. This trial was a standard 3 + 3 dose escalation with 6 patients each in cohorts 4 and 5 to gather more immunologic data. Nineteen of 21 patients enrolled had locally recurrent prostate cancer after definitive radiation therapy, and 2 had no local therapy. All cohorts received initial subcutaneous vaccination with recombinant vaccinia (rV)-PSA-TRICOM and intraprostatic booster vaccinations with recombinant fowlpox (rF)-PSA-TRICOM. Cohorts 3–5 also received intraprostatic rF-GM-CSF. Cohort 5 received additional subcutaneous boosters with rF-PSA-TRICOM and rF-GM-CSF. Patients had pre- and post-treatment prostate biopsies, and analyses of peripheral and intraprostatic immune cells were performed. There were no dose-limiting toxicities, and the maximum tolerated dose was not reached. The most common grade 2 adverse events were fever (38 %) and subcutaneous injection site reactions (33 %); the single grade 3 toxicity was transient fever. Overall, 19 of 21 patients on trial had stable (10) or improved (9) PSA values. There was a marked increase in CD4+ (p = 0.0002) and CD8+ (p = 0.0002) tumor infiltrates in post- versus pre-treatment tumor biopsies. Four of 9 patients evaluated had peripheral immune responses to PSA or NGEP. Intraprostatic administration of PSA-TRICOM is safe and feasible and can generate a significant immunologic response. Improved serum PSA kinetics and intense post-vaccination inflammatory infiltrates were seen in the majority of patients. Clinical trials examining clinical end points are warranted.


Similar content being viewed by others
References
Terasawa H, Tsang KY, Gulley J, Arlen P, Schlom J (2002) Identification and characterization of a human agonist cytotoxic T-lymphocyte epitope of human prostate-specific antigen. Clin Cancer Res 8:41–53
Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR (2010) Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 28:1099–1105. doi:10.1200/JCO.2009.25.0597
Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, Skarupa L, Jones JL, Poole DJ, Higgins JP, Hodge JW, Cereda V, Vergati M, Steinberg SM, Halabi S, Jones E, Chen C, Parnes H, Wright JJ, Dahut WL, Schlom J (2010) Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother 59:663–674. doi:10.1007/s00262-009-0782-8
Madan RA, Gulley JL, Fojo T, Dahut WL (2010) Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist 15:969–975. doi:10.1634/theoncologist.2010-0129
Madan RA, Mohebtash M, Schlom J, Gulley JL (2010) Therapeutic vaccines in metastatic castration-resistant prostate cancer: principles in clinical trial design. Expert Opin Biol Ther 10:19–28. doi:10.1517/14712590903321421
Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, Levine EG, Blumenstein BA, Vogelzang NJ (2003) Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol 21:1232–1237
Kudo-Saito C, Schlom J, Hodge JW (2005) Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res 11:2416–2426. doi:10.1158/1078-0432.CCR-04-1380
Slavin-Chiorini DC, Catalfamo M, Kudo-Saito C, Hodge JW, Schlom J, Sabzevari H (2004) Amplification of the lytic potential of effector/memory CD8 + cells by vector-based enhancement of ICAM-1 (CD54) in target cells: implications for intratumoral vaccine therapy. Cancer Gene Ther 11:665–680. doi:10.1038/sj.cgt.7700741
Hodge JW, Abrams S, Schlom J, Kantor JA (1994) Induction of antitumor immunity by recombinant vaccinia viruses expressing B7–1 or B7–2 costimulatory molecules. Cancer Res 54:5552–5555
Matzinger P (2002) The danger model: a renewed sense of self. Science 296:301–305. doi:10.1126/science.1071059
Kaufman HL, Cohen S, Cheung K, DeRaffele G, Mitcham J, Moroziewicz D, Schlom J, Hesdorffer C (2006) Local delivery of vaccinia virus expressing multiple costimulatory molecules for the treatment of established tumors. Hum Gene Ther 17:239–244. doi:10.1089/hum.2006.17.239
Gomella LG, Mastrangelo MJ, McCue PA, Maguire HJ, Mulholland SG, Lattime EC (2001) Phase I study of intravesical vaccinia virus as a vector for gene therapy of bladder cancer. J Urol 166:1291–1295
Mastrangelo MJ, Maguire HC Jr, Eisenlohr LC, Laughlin CE, Monken CE, McCue PA, Kovatich AJ, Lattime EC (1999) Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther 6:409–422. doi:10.1038/sj.cgt.7700066
Kudo-Saito C, Schlom J, Hodge JW (2004) Intratumoral vaccination and diversified subcutaneous/intratumoral vaccination with recombinant poxviruses encoding a tumor antigen and multiple costimulatory molecules. Clin Cancer Res 10:1090–1099
Marshall JL, Gulley JL, Arlen PM, Beetham PK, Tsang KY, Slack R, Hodge JW, Doren S, Grosenbach DW, Hwang J, Fox E, Odogwu L, Park S, Panicali D, Schlom J (2005) Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol 23:720–731. doi:10.1200/JCO.2005.10.206
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 26:1148–1159. doi:10.1200/JCO.2007.12.4487
Memorial Sloan-Kettering Cancer Center Prediction Tools: Prostate Cancer Nomograms. http://nomograms.mskcc.org/Prostate/PsaDoublingTime.aspx. Accessed Dec 2012
Gulley J, Chen AP, Dahut W, Arlen PM, Bastian A, Steinberg SM, Tsang K, Panicali D, Poole D, Schlom J, Michael Hamilton J (2002) Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate 53:109–117. doi:10.1002/pros.10130
Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, Tsang KY, Yokokawa J, Hodge JW, Menard C, Camphausen K, Coleman CN, Sullivan F, Steinberg SM, Schlom J, Dahut W (2005) Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res 11:3353–3362. doi:10.1158/1078-0432.CCR-04-2062
Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, Tsang KY, Poole DJ, Parnes HL, Wright JJ, Dahut WL, Schlom J, Gulley JL (2012) Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 13:501–508. doi:10.1016/S1470-2045(12)70006-2
Mannon PJ, Leon F, Fuss IJ, Walter BA, Begnami M, Quezado M, Yang Z, Yi C, Groden C, Friend J, Hornung RL, Brown M, Gurprasad S, Kelsall B, Strober W (2009) Successful granulocyte-colony stimulating factor treatment of Crohn’s disease is associated with the appearance of circulating interleukin-10-producing T cells and increased lamina propria plasmacytoid dendritic cells. Clin Exp Immunol 155:447–456. doi:10.1111/j.1365-2249.2008.03799.x
Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, Beetham P, Tsang KY, Grosenbach DW, Feldman J, Steinberg SM, Jones E, Chen C, Marte J, Schlom J, Dahut W (2006) A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res 12:1260–1269. doi:10.1158/1078-0432.CCR-05-2059
Arlen PM, Gulley JL, Todd N, Lieberman R, Steinberg SM, Morin S, Bastian A, Marte J, Tsang KY, Beetham P, Grosenbach DW, Schlom J, Dahut W (2005) Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol 174:539–546. doi:10.1097/01.ju.0000165159.33772.5b
Arlen PM, Skarupa L, Pazdur M, Seetharam M, Tsang KY, Grosenbach DW, Feldman J, Poole DJ, Litzinger M, Steinberg SM, Jones E, Chen C, Marte J, Parnes H, Wright J, Dahut W, Schlom J, Gulley JL (2007) Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol 178:1515–1520. doi:10.1016/j.juro.2007.05.117
DiPaola R, Chen Y, Bubley G, Hahn N, Stein M, Schlom J, Gulley J, Lattime E, Carducci M, Wilding G (2009) A phase II study of PROSTVAC-V (vaccinia)/TRICOM and PROSTVAC-F (fowlpox)/TRICOM with GM-CSF in patients with PSA progression after local therapy for prostate cancer: results of ECOG 9802. ASCO Genitourinary Cancers Symposium. Abstr 108. http://meetinglibrary.asco.org/content/20355-64
DiPaola RS, Plante M, Kaufman H, Petrylak DP, Israeli R, Lattime E, Manson K, Schuetz T (2006) A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7–1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in patients with prostate cancer. J Transl Med 4:1. doi:10.1186/1479-5876-4-1
Eder JP, Kantoff PW, Roper K, Xu GX, Bubley GJ, Boyden J, Gritz L, Mazzara G, Oh WK, Arlen P, Tsang KY, Panicali D, Schlom J, Kufe DW (2000) A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res 6:1632–1638
Kaufman HL, Wang W, Manola J, DiPaola RS, Ko YJ, Sweeney C, Whiteside TL, Schlom J, Wilding G, Weiner LM (2004) Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 22:2122–2132. doi:10.1200/JCO.2004.08.083
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363:411–422. doi:10.1056/NEJMoa1001294
Litzinger MT, Foon KA, Sabzevari H, Tsang KY, Schlom J, Palena C (2009) Chronic lymphocytic leukemia (CLL) cells genetically modified to express B7–1, ICAM-1, and LFA-3 confer APC capacity to T cells from CLL patients. Cancer Immunol Immunother 58:955–965. doi:10.1007/s00262-008-0611-5
Stein WD, Gulley JL, Schlom J, Madan RA, Dahut W, Figg WD, Ning YM, Arlen PM, Price D, Bates SE, Fojo T (2011) Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res 17:907–917. doi:10.1158/1078-0432.CCR-10-1762
Gulley JL, Madan RA, Schlom J (2011) Impact of tumour volume on the potential efficacy of therapeutic vaccines. Curr Oncol 18:e150–e157
Gulley JL (2013) Therapeutic vaccines: the ultimate personalized therapy? Hum Vaccin Immunother 9:219–221. doi:10.4161/hv.22106
Disis ML, Wallace DR, Gooley TA, Dang Y, Slota M, Lu H, Coveler AL, Childs JS, Higgins DM, Fintak PA, dela Rosa C, Tietje K, Link J, Waisman J, Salazar LG (2009) Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol 27:4685–4692. doi:10.1200/JCO.2008.20.6789
Hardwick N, Chain B (2011) Epitope spreading contributes to effective immunotherapy in metastatic melanoma patients. Immunotherapy 3:731–733. doi:10.2217/imt.11.62
Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, Hilf N, Schoor O, Fritsche J, Mahr A, Maurer D, Vass V, Trautwein C, Lewandrowski P, Flohr C, Pohla H, Stanczak JJ, Bronte V, Mandruzzato S, Biedermann T, Pawelec G, Derhovanessian E, Yamagishi H, Miki T, Hongo F, Takaha N, Hirakawa K, Tanaka H, Stevanovic S, Frisch J, Mayer-Mokler A, Kirner A, Rammensee HG, Reinhardt C, Singh-Jasuja H (2012) Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med doi:10.1038/nm.2883 (Epub ahead of print)
Romero P, Dunbar PR, Valmori D, Pittet M, Ogg GS, Rimoldi D, Chen JL, Lienard D, Cerottini JC, Cerundolo V (1998) Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J Exp Med 188:1641–1650
Clemente CG, Mihm MC Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N (1996) Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 77:1303–1310. doi:10.1002/(SICI)1097-0142(19960401)77:7<1303:AID-CNCR12>3.0.CO;2-5
Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, Lebecque S, Fridman WH, Cadranel J (2008) Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 26:4410–4417. doi:10.1200/JCO.2007.15.0284
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY (2007) Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 25:2586–2593. doi:10.1200/JCO.2006.09.4565
Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, Itoh T, Ohbuchi T, Kondo S, Katoh H (2006) Concurrent infiltration by CD8 + T cells and CD4 + T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer 94:275–280. doi:10.1038/sj.bjc.6602934
Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H (1998) CD8 + T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 58:3491–3494
Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ, Sato E (2007) CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA 104:3967–3972. doi:10.1073/pnas.0611618104
Shafer-Weaver KA, Watkins SK, Anderson MJ, Draper LJ, Malyguine A, Alvord WG, Greenberg NM, Hurwitz AA (2009) Immunity to murine prostatic tumors: continuous provision of T-cell help prevents CD8 T-cell tolerance and activates tumor-infiltrating dendritic cells. Cancer Res 69:6256–6264. doi:10.1158/0008-5472.CAN-08-4516
Kudo-Saito C, Garnett CT, Wansley EK, Schlom J, Hodge JW (2007) Intratumoral delivery of vector mediated IL-2 in combination with vaccine results in enhanced T cell avidity and anti-tumor activity. Cancer Immunol Immunother 56:1897–1910. doi:10.1007/s00262-007-0332-1
Kudo-Saito C, Wansley EK, Gruys ME, Wiltrout R, Schlom J, Hodge JW (2007) Combination therapy of an orthotopic renal cell carcinoma model using intratumoral vector-mediated costimulation and systemic interleukin-2. Clin Cancer Res 13:1936–1946. doi:10.1158/1078-0432.CCR-06-2398
Acknowledgments
Grant support was provided by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. The authors thank Benedetto Farsaci and Caroline Jochems for their helpful discussions, Diane J. Poole for her technical assistance, and Bonnie L. Casey and Debra Weingarten for their editorial assistance in the preparation of this manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gulley, J.L., Heery, C.R., Madan, R.A. et al. Phase I study of intraprostatic vaccine administration in men with locally recurrent or progressive prostate cancer. Cancer Immunol Immunother 62, 1521–1531 (2013). https://doi.org/10.1007/s00262-013-1448-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-013-1448-0