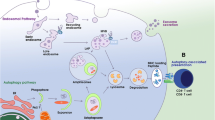
Abstract
T cells detect infected and transformed cells via antigen presentation by major histocompatibility complex (MHC) molecules on the cell surface. For T cell stimulation, these MHC molecules present fragments of proteins that are expressed or taken up by the cell. These fragments are generated by distinct proteolytic mechanisms for presentation on MHC class I molecules to cytotoxic CD8+ and on MHC class II molecules to helper CD4+ T cells. Proteasomes are primarily involved in MHC class I ligand and lysosomes, in MHC class II ligand generation. Autophagy delivers cytoplasmic material to lysosomes and, therefore, contributes to cytoplasmic antigen presentation by MHC class II molecules. In addition, it has been recently realized that this process also supports extracellular antigen processing for MHC class II presentation and cross-presentation on MHC class I molecules. Although the exact mechanisms for the regulation of these antigen processing pathways by autophagy are still unknown, recent studies, summarized in this review, suggest that they contribute to immune responses against infections and to maintain tolerance. Moreover, they are targeted by viruses for immune escape and could maybe be harnessed for immunotherapy.

Similar content being viewed by others
References
Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S (1999) SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50(3–4):213–219
Peaper DR, Cresswell P (2008) Regulation of MHC class I assembly and peptide binding. Annu Rev Cell Dev Biol 24:343–368
Hammer GE, Kanaseki T, Shastri N (2007) The final touches make perfect the peptide-MHC class I repertoire. Immunity 26(4):397–406
Bevan MJ (1976) Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med 143(5):1283–1288
Amigorena S, Savina A (2010) Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr Opin Immunol 22(1):109–117
Saveanu L, Carroll O, Weimershaus M et al (2009) IRAP identifies an endosomal compartment required for MHC class I cross-presentation. Science 325(5937):213–217
Houde M, Bertholet S, Gagnon E et al (2003) Phagosomes are competent organelles for antigen cross-presentation. Nature 425(6956):402–406
Guermonprez P, Saveanu L, Kleijmeer M et al (2003) ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425(6956):397–402
Goldszmid RS, Coppens I, Lev A et al (2009) Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J Exp Med 206(2):399–410
Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C (2007) Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 316(5824):612–616
Trombetta ES, Mellman I (2005) Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol 23:975–1028
Mizushima N, Klionsky DJ (2007) Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr 27:19–40
Yla-Anttila P, Vihinen H, Jokitalo E, Eskelinen EL (2009) 3D tomography reveals connections between the phagophore and endoplasmic reticulum. Autophagy 5(8):1180–1185
Hayashi-Nishino M, Fujita N, Noda T et al (2009) A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol 11(12):1433–1437
English L, Chemali M, Duron J et al (2009) Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol 10(5):480–487
Ohsumi Y (2001) Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol 2(3):211–216
Nakatogawa H, Ichimura Y, Ohsumi Y (2007) Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell 130(1):165–178
Thurston TL, Ryzhakov G, Bloor S, von Muhlinen N, Randow F (2009) The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat Immunol 10(11):1215–1221
Zheng YT, Shahnazari S, Brech A et al (2009) The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J Immunol 183(9):5909–5916
Ponpuak M, Davis AS, Roberts EA et al (2010) Delivery of cytosolic components by p62 endows autophagosomes with unique anti-microbial properties. Immunity 32:329–341
Itakura E, Kishi C, Inoue K, Mizushima N (2008) Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 19(12):5360–5372
Matsunaga K, Saitoh T, Tabata K et al (2009) Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol 11(4):385–396
Zhong Y, Wang QJ, Li X et al (2009) Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol 11(4):468–476
Liang C, Feng P, Ku B et al (2006) Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol 8(7):688–699
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451(7182):1069–1075
Schmid D, Pypaert M, Münz C (2007) MHC class II antigen loading compartments continuously receive input from autophagosomes. Immunity 26:79–92
Dengjel J, Schoor O, Fischer R et al (2005) Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci USA 102:7922–7927
Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L (2008) Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature 455(7211):396–400
Kasai M, Tanida I, Ueno T et al (2009) Autophagic compartments gain access to the MHC class II compartments in thymic epithelium. J Immunol 183(11):7278–7285
Zhou D, Li P, Lott JM et al (2005) Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity 22:571–581
Lee DY, Sugden B (2008) The latent membrane protein 1 oncogene modifies B-cell physiology by regulating autophagy. Oncogene 27(20):2833–2842
Paludan C, Schmid D, Landthaler M et al (2005) Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 307(5709):593–596
Leung CS, Haigh TA, Mackay LK, Rickinson AB, Taylor GS (2010) Nuclear location of an endogenously expressed antigen, EBNA1, restricts access to macroautophagy and the range of CD4 epitope display. Proc Natl Acad Sci USA 107(5):2165–2170
Riedel A, Nimmerjahn F, Burdach S et al (2008) Endogenous presentation of a nuclear antigen on MHC class II by autophagy in the absence of CRM1-mediated nuclear export. Eur J Immunol 38(8):2090–2095
Leib DA, Alexander DE, Cox D, Yin J, Ferguson TA (2009) Interaction of ICP34.5 with Beclin 1 modulates herpes simplex virus type 1 pathogenesis through control of CD4+ T-cell responses. J Virol 83(23):12164–12171
Lee HK, Mattei LM, Steinberg BE et al (2010) In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity 32(2):227–239
Jagannath C, Lindsey DR, Dhandayuthapani S et al (2009) Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med 15(3):267–276
Dorfel D, Appel S, Grunebach F et al (2005) Processing and presentation of HLA class I and II epitopes by dendritic cells after transfection with in vitro transcribed MUC1 RNA. Blood 105(8):3199–3205
Blander JM, Medzhitov R (2006) Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440(7085):808–812
Sanjuan MA, Dillon CP, Tait SW et al (2007) Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 450(7173):1253–1257
Cooney R, Baker J, Brain O et al (2010) NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med 16(1):90–97
Travassos LH, Carneiro LA, Ramjeet M et al (2009) Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol 11(1):55–62
Blanchet FP, Moris A, Nikolic DS et al (2010) Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity 32:654–669
Li Y, Wang LX, Yang G et al (2008) Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res 68(17):6889–6895
Uhl M, Kepp O, Jusforgues-Saklani H et al (2009) Autophagy within the antigen donor cell facilitates efficient antigen cross-priming of virus-specific CD8+ T cells. Cell Death Differ 16(7):991–1005
Orvedahl A, Alexander D, Talloczy Z et al (2007) HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 1:23–35
Liang XH, Kleeman LK, Jiang HH et al (1998) Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol 72(11):8586–8596
Orvedahl A, MacPherson S, Sumpter R Jr et al (2010) Autophagy protects against Sindbis virus infection of the central nervous system. Cell Host Microbe 7(2):115–127
Shelly S, Lukinova N, Bambina S, Berman A, Cherry S (2009) Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity 30(4):588–598
Talloczy Z, Jiang W (2002) Virgin HWt, et al. Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc Natl Acad Sci USA 99(1):190–195
Alexander DE, Ward SL, Mizushima N, Levine B, Leib DA (2007) Analysis of the role of autophagy in replication of herpes simplex virus in cell culture. J Virol 81(22):12128–12134
Chaumorcel M, Souquere S, Pierron G, Codogno P, Esclatine A (2008) Human cytomegalovirus controls a new autophagy-dependent cellular antiviral defense mechanism. Autophagy 4(1):46–53
Pattingre S, Tassa A, Qu X et al (2005) Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122(6):927–939
Lee JS, Li Q, Lee JY et al (2009) FLIP-mediated autophagy regulation in cell death control. Nat Cell Biol 11(11):1355–1362
Ku B, Woo JS, Liang C et al (2008) Structural and biochemical bases for the inhibition of autophagy and apoptosis by viral BCL-2 of murine gamma-herpesvirus 68. PLoS Pathog 4(2):e25
Xiaofei E, Hwang S, Oh S et al (2009) Viral Bcl-2-mediated evasion of autophagy aids chronic infection of gammaherpesvirus 68. PLoS Pathog 5(10):e1000609
Dales S, Eggers HJ, Tamm I, Palade GE (1965) Electron microscopic study of the formation of poliovirus. Virology 26:379–389
Jackson WT, Giddings TH Jr, Taylor MP et al (2005) Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol 3(5):e156
Sir D, Chen WL, Choi J et al (2008) Induction of incomplete autophagic response by hepatitis C virus via the unfolded protein response. Hepatology 48(4):1054–1061
Dreux M, Gastaminza P, Wieland SF, Chisari FV (2009) The autophagy machinery is required to initiate hepatitis C virus replication. Proc Natl Acad Sci USA 106(33):14046–14051
Lee YR, Lei HY, Liu MT et al (2008) Autophagic machinery activated by dengue virus enhances virus replication. Virology 374(2):240–248
Khakpoor A, Panyasrivanit M, Wikan N, Smith DR (2009) A role for autophagolysosomes in dengue virus 3 production in HepG2 cells. J Gen Virol 90(Pt 5):1093–1103
Panyasrivanit M, Khakpoor A, Wikan N, Smith DR (2009) Co-localization of constituents of the dengue virus translation and replication machinery with amphisomes. J Gen Virol 90(Pt 2):448–456
Kyei GB, Dinkins C, Davis AS et al (2009) Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol 186(2):255–268
Zhou Z, Jiang X, Liu D et al (2009) Autophagy is involved in influenza A virus replication. Autophagy 5(3):321–328
Gannage M, Dormann D, Albrecht R et al (2009) Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell Host Microbe 6(4):367–380
Acknowledgements
Our research is supported by the National Cancer Institute (R01CA108609 and R01CA101741), the Foundation for the National Institutes of Health (Grand Challenges in Global Health), and the Swiss National Science Foundation (310030_126995).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of the Special Issue on Autophagy.
Rights and permissions
About this article
Cite this article
Gannage, M., Münz, C. MHC presentation via autophagy and how viruses escape from it. Semin Immunopathol 32, 373–381 (2010). https://doi.org/10.1007/s00281-010-0227-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-010-0227-7