Abstract
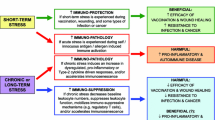
Animals receive environmental stimuli from neural signals in order to produce hormones that control immune responses. Glucocorticoids (GCs) are a group of steroid hormones produced in the adrenal cortex and well-known mediators for the nervous and immune systems. GC secretion is induced by circadian rhythm and stress, and plasma GC levels are high at the active phase of animals and under stress condition. Clinically, GCs are used for allergies, autoimmunity, and chronic inflammation, because they have strong anti-inflammatory effects and induce the apoptosis of lymphocytes. Glucocorticoid receptor (GR) acts as a transcription factor and represses the expression of inflammatory cytokines, chemokines, and prostaglandins by binding to its motif, glucocorticoid-response element, or to other transcription factors. In mice, GR suppresses the antigen-stimulated inflammation mediated by macrophages, dendritic cells, and epithelial cells, and impairs cytotoxic immune responses by downregulating interferon-γ production and inhibiting the development of type-1 helper T cells, CD8+ T cells, and natural killer cells. These immune inhibitory effects prevent lethality by excessive inflammation, but at the same time increase the susceptibility to infection and cancer. GCs can also activate the immune system. The circadian cycle of GC secretion controls the diurnal oscillations of the distribution and response of T cells, thus supporting T cell maintenance and effective immune protection against infection. Moreover, several reports have shown that GR has the potential to enhance the activities of Th2, Th17, and immunoglobulin-producing B cells. Stress has two different effects on immune responses: immune suppression to cause mortality by infection and cancer, and excessive immune activation to induce chronic inflammation and autoimmune disease. Consistently, stress-induced GCs strongly suppress cell-mediated immunity and cause viral infection and tumor development. They may also enhance the development of pathogenic helper T cells and cause tissue damage through neural and intestinal inflammation. Past studies have reported the positive and negative effects of GCs on the immune system. These opposing properties of GCs may regulate the immune balance between the responsiveness to antigens and excessive inflammation in steady-state and stress conditions.

Similar content being viewed by others
References
Cain DW, Cidlowski JA (2017) Immune regulation by glucocorticoids. Nat Rev Immunol 17:233–247
Vandevyver S, Dejager L, Libert C (2014) Comprehensive overview of the structure and regulation of the glucocorticoid receptor. Endocr Rev 35:671–693
Almawi WY, Beyhum HN, Rahme AA, Rieder MJ (1996) Regulation of cytokine and cytokine receptor expression by glucocorticoids. J Leukoc Biol 60:563–572
Kunicka JE, Talle MA, Denhardt GH, Brown M, Prince LA, Goldstein G (1993) Immunosuppression by glucocorticoids: inhibition of production of multiple lymphokines by in vivo administration of dexamethasone. Cell Immunol 149:39–49
Fushimi T, Shimura S, Suzuki S, Saitoh H, Okayama H, Shirato K (1998) Suppression of gene expression and production of interleukin 13 by dexamethasone in human peripheral blood mononuclear cells. Tohoku J Exp Med 185:157–160
Rolfe FG, Hughes JM, Armour CL, Sewell WA (1992) Inhibition of interleukin-5 gene expression by dexamethasone. Immunology 77:494–499
Brewer JA, Kanagawa O, Sleckman BP, Muglia LJ (2002) Thymocyte apoptosis induced by T cell activation is, mediated by glucocorticoids in vivo. J Immunol 169:1837–1843
Surjit M, Ganti KP, Mukherji A, Ye T, Hua G, Metzger D, Li M, Chambon P (2011) Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell 145:224–241
Auphan N, Didonato JA, Rosette C, Helmberg A, Karin M (1995) Immunosuppression by glucocorticoids: inhibition of NF-kB activity through induction of IkB synthesis. Science 270:286–290
Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, Saklatvala J, Clark AR (2006) Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med 203:1883–1889
Berrebi D, Bruscoli S, Cohen N, Foussat A, Migliorati G, Bouchet-Delbos L, Maillot MC, Portier A, Couderc J, Galanaud P, Peuchmaur M, Riccardi C, Emilie D (2003) Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: an anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10. Blood 101:729–738
Smoak K, Cidlowski JA (2006) Glucocorticoids regulate tristetraprolin synthesis and posttranscriptionally regulate tumor necrosis factor alpha inflammatory signaling. Mol Cell Biol 2006(26):9126–9135
Tobler A, Meier R, Seitz M, Dewald B, Baggiolini M, Fey MF (1992) Glucocorticoids downregulate gene expression of GM-CSF, NAP-1/IL-8, and IL-6, but not of M-CSF in human fibroblasts. Blood 79:45–51
Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS (1995) Characterization of mechanisms involved in transrepression of NF-kB by activated glucocorticoid receptors. Mol Cell Biol 15:943–953
Jonat C, Rahmsdorf HJ, Park KK, Cato ACB, Gebel S, Ponta H, Herrlich P (1990) Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell 62:1189–1204
Newton R, Kuitert LM, Slater DM, Adcock IM, Barnes PJ (1996) Cytokine induction of cytosolic phospholipase A2 and cyclooxygenase-2 mRNA is suppressed by glucocorticoids in human epithelial cells. Life Sci 60:67–78
Lin LL, Lin AY, Dewitt DL (1992) Interleukin-1a induces the accumulation of cytosolic phospholipase A2 and the release of prostaglandin E2 in human fibroblasts. J Biol Chem 267:23451–23454
Hoeck WG, Ramesha CS, Chang DJ, Fan N, Heller RA (1993) Cytoplasmic phospholipase A2 activity and gene expression are stimulated by tumor necrosis factor: dexamethasone blocks the induced synthesis. Proc Natl Acad Sci U S A 90:4475–4479
Masferrer JL, Zweifel BS, Seibert K, Needleman P (1990) Selective regulation of cellular cyclooxygenase by dexamethasone and endotoxin in mice. J Clin Invest 1990(86):1375–1379
Rider LG, Hirasawa N, Santini F, Beaven MA (1996) Activation of the mitogen activated protein kinase cascade is suppressed by low concentrations of dexamethasone in mast cells. J Immunol 157:2374–2380
Kleiman A, Hubner S, Parkitna JMR, Neumann A, Hofer S, Weigand MA, Bauer M, Schmid W, Schutz G, Libert C, Reichardt HM, Tuckermann JP (2012) Glucocorticoid receptor dimerization is required for survival in septic shock via suppression of interleukin-1 in macrophages. FASEB J 26:722–729
Tuckermann JP, Kleiman A, Moriggl R, Spanbroek R, Neumann A, Illing A, Clausen BE, Stride B, Forster I, Habenicht AJ, Reichardt HM, Tronche F, Schmid W, Schutz G (2007) Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J Clin Invest 117:1381–1390
Klassen C, Karabinskaya A, Dejager L, Vettorazzi S, Van Moorleghem J, Luhder F, Meijsing SH, Tuckermann JP, Bohnenberger H, Libert C, Reichardt HM (2017) Airway epithelial cells are crucial targets of glucocorticoids in a mouse model of allergic asthma. J Immunol 199:48–61
Baschant U, Frappart L, Rauchhaus U, Bruns L, Reichardt HM, Kamradt T, Brauer R, Tuckermann JP (2011) Glucocorticoid therapy of antigen induced arthritis depends on the dimerized glucocorticoid receptor in T cells. Proc Natl Acad Sci U S A 108:19317–19322
Siegel RM, Katsumata M, Miyashita T, Louie DC, Greene MI, Reed JC (1992) Inhibition of thymocyte apoptosis and negative antigenic selection in Bcl-2 transgenic mice. Proc Natl Acad Sci U S A 89:7003–7007
Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P (1998) Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell 94:727–737
Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, Penninger JM, Mak TW (1998) Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 94:739–750
Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, Elia A, de la Pompa JL, Kagi D, Khoo W, Potter J, Yoshida R, Kaufman SA, Lowe SW, Penninger JM, Mak TW (1998) Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell 94:339–352
Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MSS, Rakic P, Flavell RA (1998) Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94:325–337
Dieken ES, Miesfeld RL (1992) Transcriptional transactivation functions localized to the glucocorticoid receptor N terminus are necessary for steroid induction of lymphocyte apoptosis. Mol Cell Biol 12:589–597
Gibbs J, Ince L, Matthews L, Mei JJ, Bell T, Yang N, Saer B, Begley N, Poolman T, Pariollaud M, Farrow S, DeMayo F, Hussell T, Worthen GS, Ray D, Loudon A (2014) An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med 20:919–926
Ince LM, Zhang ZG, Beesley S, Vonslow RM, Saer B, Matthews LC, Begley N, Gibbs JE, Ray DW, Loudon ASI (2019) Circadian variation in pulmonary inflammatory responses is independent of rhythmic glucocorticoid signaling in airway epithelial cells. FASEB J 33:126–139
Bhattacharyya S, Brown DE, Brewer JA, Vogt SK, Muglia LJ (2007) Macrophage glucocorticoid receptors regulate toll-like receptor 4-mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood 109:4313–4319
Li CYC, Munitic I, Mittelstadt PR, Castro E, Ashwell JD (2015) Suppression of dendritic cell-derived IL-12 by endogenous glucocorticoids is protective in LPS-induced sepsis. PLoS Biol 13:e1002269
Quatrini L, Wieduwild E, Guia S, Bernat C, Glaichenhaus N, Vivier E, Ugolini S (2017) Host resistance to endotoxic shock requires the neuroendocrine regulation of group 1 innate lymphoid cells. J Exp Med 214:3531–3541
Quatrini L, Wieduwild E, Escaliere B, Filtjens J, Chasson L, Laprie C, Vivier E, Ugolini S (2018) Endogenous glucocorticoids control host resistance to viral infection through the tissue-specific regulation of PD-1 expression on NK cells. Nat Immunol 19:954–962
Chen LY, Jondal M, Yakimchuk K (2018) Regulatory effects of dexamethasone on NK and T cell immunity. Inflammopharmacology 26:1331–1338
Brewer JA, Khor B, Vogt SK, Muglia LM, Fujiwara H, Haegele KE, Sleckman BP, Muglia LJ (2003) T cell glucocorticoid receptor is required to suppress COX-2-mediated lethal immune activation. Nat Med 9:1318–1322
Wu CY, Wang KN, McDyer JF, Seder RA (1998) Prostaglandin E2 and dexamethasone inhibit IL-12 receptor expression and IL-12 responsiveness. J Immunol 161:2723–2730
Franchimont D, Galon J, Gadina M, Visconti R, Zhou YJ, Aringer M, Frucht DM, Chrousos GP, O’Shea JJ (2000) Inhibition of Th1 immune response by glucocorticoids, dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. J Immunol 164:1768–1774
Liberman AC, Refojo D, Druker J, Toscano M, Rein T, Holsboer F, Arzt E (2007) The activated glucocorticoid receptor inhibits the transcription factor T-bet by direct protein-protein interaction. FASEB J 21:1177–1188
Kugler DG, Mittelstadt PR, Ashwell JD, Sher A, Jankovic D (2013) CD4+ T cells are trigger and target of the glucocorticoid response that prevents lethal immunopathology in toxoplasma infection. J Exp Med 210:1919–1927
Franchimont D, Galon J, Vacchio MS, Fan S, Visconti R, Frucht DM, Geenen V, Chrousos GP, Ashwell JD, O’Shea JJ (2002) Positive effects of glucocorticoids on T cell function by up-regulation of IL-7 receptor α. J Immunol 168:2212–2218
Diefenbach A, Colonna M, Koyasu S (2014) Development, differentiation, and diversity of innate lymphoid cells. Immunity 41:354–365
Mazzucchelli R, Durum SK (2007) Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol 7:144–154
Tani-ichi S, Shimba A, Wagatsuma K, Miyachi H, Kitano S, Imai K, Hara T, Ikuta K (2013) Interleukin-7 receptor controls development and maturation of late stages of thymocyte subpopulations. Proc Natl Acad Sci U S A 110:612–617
Lee HC, Shibata H, Ogawa S, Maki K, Ikuta K (2005) Transcriptional regulation of the mouse IL-7 receptor α promoter by glucocorticoid receptor. J Immunol 174:7800–7806
Abe A, Tani-ichi S, Shitara S, Cui G, Yamada H, Miyachi H, Kitano S, Hara T, Abe R, Yoshikai Y, Ikuta K (2015) An enhancer of the IL-7 receptor α-chain locus controls IL-7 receptor expression and maintenance of peripheral T cells. J Immunol 195:3129–3138
Shimba A, Cui GW, Tani-ichi S, Ogawa M, Abe S, Okazaki F, Kitano S, Miyachi H, Yamada H, Hara T, Yoshikai Y, Nagasawa T, Schutz G, Ikuta K (2018) Glucocorticoids drive diurnal oscillations in T cell distribution and responses by inducing interleukin-7 receptor and CXCR4. Immunity 48:286–298
Haus E, Smolensky MH (1999) Biologic rhythms in the immune system. Chronobiol Int 16:581–622
Dimitrov S, Benedict C, Heutling D, Westermann J, Born J, Lange T (2009) Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood 113:5134–5143
Jourdan P, Vendrell JP, Huguet MF, Segondy M, Bousquet J, Pene J, Yssel H (2000) Cytokines and cell surface molecules independently induce CXCR4 expression on CD4+ CCR7+ human memory T cells. J Immunol 165:716–724
Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LAJ (2014) Circadian clock proteins and immunity. Immunity 40:178–186
Suzuki K, Hayano Y, Nakai A, Furuta F, Noda M (2016) Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. J Exp Med 213:2567–2574
Druzd D, Matveeva O, Ince L, Harrison U, He WY, Schmal C, Herzel H, Tsang AH, Kawakami N, Leliavski A, Uhl O, Yao L, Sander LE, Chen CS, Kraus K, de Juan A, Hergenhan SM, Ehlers M, Koletzko B, Haas R, Solbach W, Oster H, Scheiermann C (2017) Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity 46:120–132
Erlacher M, Knoflach M, Stec IEM, Bock G, Wick G, Wiegers GJ (2005) TCR signaling inhibits glucocorticoid-induced apoptosis in murine thymocytes depending on the stage of development. Eur J Immunol 35:3287–3296
Bellet MM, Deriu E, Liu JZ, Grimaldi B, Blaschitz C, Zeller M, Edwards RA, Sahar S, Dandekar S, Baldi P, George MD, Raffatellu M, Sassone-Corsi P (2013) Circadian clock regulates the host response to Salmonella. Proc Natl Acad Sci U S A 110:9897–9902
Grayson J, Dooley NJ, Koski IR, Blaese RM (1981) Immunoglobulin production induced in vitro by glucocorticoid hormones: T cell-dependent stimulation of immunoglobulin production without B cell proliferation in cultures of human peripheral blood lymphocytes. J Clin Invest 68:1539–1547
Lechner O, Wiegers GJ, Oliveira-dos-Santos AJ, Dietrich H, Recheis H, Waterman M, Boyd R, Wick G (2000) Glucocorticoid production in the murine thymus. Eur J Immunol 30:337–346
Cima I, Corazza N, Dick B, Fuhrer A, Herren S, Jakob S, Ayuni E, Mueller C, Brunner T (2004) Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J Exp Med 200:1635–1646
Mukherji A, Kobiita A, Ye T, Chambon P (2013) Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 153:812–827
Mittelstadt PR, Monteiro JP, Ashwell JD (2012) Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J Clin Invest 122:2384–2394
Cain DW, Bortner CD, Diaz-Jimenez D, Petrillo MG, Gruver-Yates A, Cidlowski JA (2020) Murine glucocorticoid receptors orchestrate B cell migration selectively between bone marrow and blood. J Immunol 205:619–629
Elenkov IJ (2004) Glucocorticoids and the Th1/Th2 balance. Ann N Y Acad Sci 1024:138–146
Blotta MH, DeKruyff RH, Umetsu DT (1997) Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J Immunol 158:5589–5595
Kodama M, Takahashi H, Iwagaki H, Itoh H, Morichika T, Yoshida A, Yoshioka H, Morimoto Y, Nishibori M, Tanaka N (2002) Effect of steroids on lipopolysaccharide/interleukin-2 induced interleukin-18 production in peripheral blood mononuclear cells. J Int Med Res 30:144–160
DeKruyff R, Fang Y, Umetsu DT (1998) Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol 160:2231–2237
Ramirez F, Fowell DJ, Puklavec M, Simmonds S, Mason D (1996) Glucocorticoids promote a Th2 cytokine response by CD4+ T cells in vitro. J Immunol 156:2406–2412
Kashiwada M, Cassel SL, Colgan JD, Rothman PB (2011) NFIL3/E4BP4 controls type 2 T helper cell cytokine expression. EMBO J 30:2071–2082
So AYL, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ (2009) Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci U S A 106:17582–17587
Nakayama T, Yamashita M (2008) Initiation and maintenance of Th2 cell identity. Curr Opin Immunol 20:265–271
Endo Y, Hirahara K, Yagi R, Tumes DJ, Nakayama T (2014) Pathogenic memory type Th2 cells in allergic inflammation. Trends Immunol 35:69–78
Yang JF, Sundrud MS, Skepner J, Yamagata T (2014) Targeting Th17 cells in autoimmune diseases. Trends Pharmacol Sci 35:493–500
Banuelos J, Cao Y, Shin SC, Lu NZ (2017) Immunopathology alters Th17 cell glucocorticoid sensitivity. Allergy 72:331–341
Hino R, Kabashima R, Kawakami C, Sugita K, Nakamura M, Tokura Y (2011) Circulating Th17 cell fluctuation in psoriatic patients treated with topical calcipotriol and betamethasone butyrate propionate. J Eur Acad Dermatol Venereol 25:243–244
Lovato P, Norsgaard H, Tokura Y, Ropke MA (2017) Calcipotriol and betamethasone dipropionate exert additive inhibitory effects on the cytokine expression of inflammatory dendritic cell-Th17 cell axis in psoriasis. J Dermatol Sci 85:147–147
Prado C, de Paz B, Gomez J, Lopez P, Rodriguez-Carrio J, Suarez A (2011) Glucocorticoids enhance Th17/Th1 imbalance and signal transducer and activator of transcription 3 expression in systemic lupus erythematosus patients. Rheumatology 50:1794–1801
Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, McCauley JL, Abreu MT, Unutmaz D, Sundrud MS (2014) Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med 211:89–104
Paugh SW, Bonten EJ, Savic D, Ramsey LB, Thierfelder WE, Gurung P, Malireddi RKS, Actis M, Mayasundari A, Min J (2015) NALP3 inflammasome upregulation and CASP1 cleavage of the glucocorticoid receptor cause glucocorticoid resistance in leukemia cells. Nat Genet 47:607–614
Schewitz-Bowers LP, Lait PJP, Copland DA, Chen P, Wu WT, Dhanda AD, Vistica BP, Williams EL, Liu BY, Jawad S, Li Z, Tucker W, Hirani S, Wakabayashi Y, Zhu J, Sen N, Conway-Campbell BL, Gery I, Dick AD, Wei L, Nussenblatt RB, Lee RW (2015) Glucocorticoid-resistant Th17 cells are selectively attenuated by cyclosporine a. Proc Natl Acad Sci U S A 112:4080–4085
Zhao J, Lloyd CM, Noble A (2013) Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol 6:335–346
Kroner JD, Steiger J, Fabri M (2018) Glucocorticoids promote intrinsic human Th17 differentiation. J Invest Dermatol 138:S11–S11
Farrell RJ, Kelleher D (2003) Glucocorticoid resistance in inflammatory bowel disease. J Endocrinol 178:339–346
Hirahara K, Mato N, Hagiwara K, Nakayama T (2018) The pathogenicity of IL-33 on steroid-resistant eosinophilic inflammation via the activation of memory-type ST2+CD4+ T cells. J Leukoc Biol 104:895–901
Karagiannidis C, Akdis M, Holopainen P, Woolley NJ, Hense G, Ruckert B, Mantel PY, Menz G, Akdis CA, Blaser K, Schmidt-Weber CB (2004) Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol 114:1425–1433
Ugor E, Prenek L, Pap R, Berta G, Ernszt D, Najbauer J, Nemeth P, Boldizsar F, Berki T (2018) Glucocorticoid hormone treatment enhances the cytokine production of regulatory T cells by upregulation of Foxp3 expression. Immunobiology 223:422–431
Chen X, Murakami T, Oppenheim JJ, Howard DMZ (2004) Differential response of murine CD4+CD25+ and CD4+CD25− T cells to dexamethasone-induced cell death. Eur J Immunol 34:859–869
Rocamora-Reverte L, Tuzlak S, von Raffay L, Tisch M, Fiegl H, Drach M, Reichardt HM, Villunger A, Tischner D, Wiegers GJ (2019) Glucocorticoid receptor-deficient Foxp3+ regulatory T cells fail to control experimental inflammatory bowel disease. Front Immunol 10:472
Tischner D, Gaggl I, Peschel I, Kaufmann M, Tuzlak S, Drach M, Thuille N, Villunger A, Wiegers GJ (2012) Defective cell death signalling along the Bcl-2 regulated apoptosis pathway compromises Treg cell development and limits their functionality in mice. J Autoimmun 38:59–69
Chen X, Oppenheim JJ, Winkler-Pickett RT, Ortaldo JR, Howard OMZ (2006) Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3+CD4+CD25+ T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur J Immunol 36:2139–2149
Sbiera S, Dexneit T, Reichardt SD, Michel KD, van den Brandt J, Schmull S, Kraus L, Beyer M, Mlynski R, Wortmann S, Allolio B, Reichardt HM, Fassnacht M (2011) Influence of short-term glucocorticoid therapy on regulatory T cells in vivo. PLoS One 6(9):e24345
Stock P, Akbari O, DeKruyff RH, Umetsu DT (2005) Respiratory tolerance is inhibited by the administration of corticosteroids. J Immunol 175:7380–7387
Wust S, van den Brandt J, Tischner D, Kleiman A, Tuckermann JP, Gold R, Luhder F, Reichardt HM (2008) Peripheral T cells are the therapeutic targets of glucocorticoids in experimental autoimmune encephalomyelitis. J Immunol 180:8434–8443
Reiche EMV, Morimoto HK, Nunes SOV (2006) Stress and depression-induced immune dysfunction: implications for the development and progression of cancer. Int Rev Psychiatry 17:515–527
Bonneau RH, Sheridan JF, Feng N, Glaser R (1991) Stress-induced suppression of herpes simplex virus (HSV)-specific cytotoxic T lymphocyte and natural killer cell activity and enhancement of acute pathogenesis following local HSV infection. Brain Behav Immun 1991(5):170–192
Steelman AJ, Dean DD, Young CR, Smith R, Prentice TW, Meagher MW, Welsh CJR (2009) Restraint stress modulates virus specific adaptive immunity during acute Theiler’s virus infection. Brain Behav Immun 23:830–843
Elftman MD, Norbury CC, Bonneau RH, Truckenmiller ME (2007) Corticosterone impairs dendritic cell maturation and function. Immunology 122:279–290
Elftman MD, Hunzeker JT, Mellinger JC, Bonneau RH, Norbury CC, Truckenmiller ME (2010) Stress-induced glucocorticoids at the earliest stages of herpes simplex virus-1 infection suppress subsequent antiviral immunity, implicating impaired dendritic cell function. J Immunol 184:1867–1875
Reiche EMV, Nunes SOV, Morimoto HK (2004) Stress, depression, the immune system, and cancer. Lancet Oncol 5:617–625
De Lorenzo BHP, Brito R, Leal TP, Garcia NP, dos Santos RMM, Alvares-Saraiva AM, Hurtado ECP, dos Reis TCB, Palma BD (2018) Chronic sleep restriction impairs the antitumor immune response in mice. Neuroimmunomodulation 2018(25):59–67
Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G (1999) Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer 80:880–888
Rosenne E, Sorski L, Shaashua L, Neeman E, Matzner P, Levi B, Ben-Eliyahu S (2014) In vivo suppression of NK cell cytotoxicity by stress and surgery: glucocorticoids have a minor role compared to catecholamines and prostaglandins. Brain Behav Immun 37:207–219
Dhabhar FS (2009) Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation 16:300–317
Busillo JM, Azzam KM, Cidlowski JA (2011) Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J Biol Chem 286:38703–38713
Wiegers GJ, Reul J (1998) Induction of cytokine receptors by glucocorticoids: functional and pathological significance. Trends Pharmacol Sci 19:317–321
Sekiyama A, Ueda H, Kashiwamura S, Nishida K, Kawai K, Teshima-kondo S, Rokutan K, Okamura H (2005) IL-18; a cytokine translates a stress into medical science. J Med Investig 52(Suppl):236–239
Qiu BS, Vallance BA, Blennerhassett PA, Collins SM (1999) The role of CD4+ lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat Med 5:1178–1182
Marchetti B, Morale MC, Testa N, Tirolo C, Caniglia S, Amor S, Dijkstra CD, Barden N (2001) Stress, the immune system and vulnerability to degenerative disorders of the central nervous system in transgenic mice expressing glucocorticoid receptor antisense RNA. Brain Res Rev 37:259–272
Arima Y, Ohki T, Nishikawa N, Higuchi K, Ota M, Tanaka Y, Nio-Kobayashi J, Elfeky M, Sakai R, Mori Y, Kawamoto T, Stofkova A, Sakashita Y, Morimoto Y, Kuwatani M, Iwanaga Y, Yoshioka Y, Sakamoto N, Yoshimura A, Takiguchi M, Sakoda S, Prinz M, Kamimura D, Murakami M (2017) Brain micro-inflammation at specific vessels dysregulates organ-homeostasis via the activation of a new neural circuit. eLife 6:e25517
Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, Nishihara M, Iwakura Y, Hirano T (2008) Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 29:628–636
Rosenberger PH, Ickovics JR, Epel E, Nadler E, Jokl P, Fulkerson JP, Tillie JM, Dhabhar FS (2009) Surgical stress-induced immune cell redistribution profiles predict short-term and long-term postsurgical recovery. J Bone Joint Surg Am 91A:2783–2794
Dhabhar FS, Satoskar AR, Bluethmann H, David JR, McEwen BS (2000) Stress-induced enhancement of skin immune function: a role for γ interferon. Proc Natl Acad Sci U S A 97:2846–2851
Dhabhar FS, Miller AH, McEwen BS, Spencer RL (1995) Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J Immunol 154:5511–5527
Miller AH, Spencer RL, Hassett J, Kim C, Rhee R, Ciurea D, Dhabhar F, McEwen B, Stein M (1994) Effects of selective type-I and type-II adrenal-steroid agonists on immune cell distribution. Endocrinology 135:1934–1944
Dhabhar FS, McEwen BS (1999) Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci U S A 96:1059–1064
Funding
This work was supported by JSPS KAKENHI Grant Numbers 16K15288 and 20K21525 (KI) and 18K15184 and 20K16280 (AS), and by the Joint Usage/Research Center program of Institute for Frontier Life and Medical Sciences Kyoto University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article is a contribution to the special issue on: Neuro-immune Interactions - Guest Editor: David Farrar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shimba, A., Ikuta, K. Control of immunity by glucocorticoids in health and disease. Semin Immunopathol 42, 669–680 (2020). https://doi.org/10.1007/s00281-020-00827-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-020-00827-8