Abstract
Background
Tumor-associated macrophages (TAMs) of the M2 phenotype are known to promote tumor proliferation and to be associated with a poor prognosis in numerous cancers. Here, we investigated whether M2 macrophages participate in the development of peritoneal dissemination in gastric cancer.
Methods
The characteristics of peritoneal macrophages in gastric cancer patients with or without peritoneal dissemination were examined by flow cytometry and the real-time quantitative polymerase chain reaction. The effects of M2 macrophages on phenotypic changes of the gastric cancer cell line MKN45 were assessed with a direct or indirect co-culture system in vitro and an in vivo mouse xenograft model.
Results
The number of peritoneal macrophages with the M2 phenotype (CD68+CD163+ or CD68+CD204+) was significantly higher in gastric cancer patients with peritoneal dissemination than in those without peritoneal dissemination. Higher expression of the M2-related messenger RNAs (IL-10, vascular endothelial growth factor A, vascular endothelial growth factor C, matrix metalloproteinase 1, and amphiregulin) and lower expression of M1-related messenger RNAs (TNF-α, CD80, CD86, and IL-12p40) were also confirmed in the TAMs. Macrophage co-culture with gastric cancer cells converted M1 phenotype into M2 phenotype. Moreover, the coexistence of MKN45 cells with M2 macrophages resulted in cancer cell proliferation and an acceleration of tumor growth in the xenograft model.
Conclusions
Intraperitoneal TAMs in gastric cancer patients with peritoneal dissemination were polarized to the M2 phenotype, and could contribute to tumor proliferation and progression. Therefore, intraperitoneal TAMs are expected to be a promising target in the treatment of peritoneal dissemination in gastric cancer.
Similar content being viewed by others
Introduction
Globally, gastric cancer is one of the commonest cancers and a frequent cause of cancer-related deaths [1]. Peritoneal dissemination is an aggravated type of metastasis in gastric cancer. Although various approaches have been assessed for the treatment of peritoneal dissemination, including systemic chemotherapy, intraperitoneal chemotherapy [2, 3], extensive intraoperative peritoneal lavage [4], and aggressive surgery [5], sufficiently satisfactory outcomes have not yet been achieved [6, 7]; this indicates the need for novel therapeutic strategies.
The peritoneal cavity is a large free space in the human body and contains a high number of cells. The connections between tumor cells and their surrounding tumor microenvironment play important roles in tumor initiation and progression. The tumor microenvironment consists of many different kinds of cells, including endothelial cells, fibroblasts, lymphocytes, and macrophages [8–11]. We have previously reported that human peritoneal mesothelial cells activated by transforming growth factor (TGF)-β1 contribute to tumor progression and fibrosis in gastric cancer [12]. Furthermore, fibrocytes derived from bone marrow play an important role in the microenvironment of tumor sites, enhancing tumor proliferation [13]. Targeting the tumor microenvironment is essential for controlling tumor development and metastasis. In the present study, we focused on macrophages. Tumor-associated macrophages (TAMs) can be classified into two phenotypes: M1 (classically activated macrophages) and M2 (alternatively activated macrophages). M2 macrophages are oriented toward promoting tumor growth, remodeling tissue, promoting angiogenesis, and suppressing adaptive immunity [14, 15]. Recently, several studies have reported that the density of M2 macrophages is associated with poor prognosis in patients with glioma, lymphoma, renal cell carcinoma, and intrahepatic cholangiocarcinoma [16–19].
TAM infiltration into gastric cancer tissue has been found to be positively correlated with the depth of invasion, nodal status, and clinical stage [20]. However, few reports have described the role of macrophages in the peritoneal dissemination of gastric cancer. High numbers of M2 macrophages in ascites were reported to be correlated with poor prognosis in patients with ovarian cancer [21]. Consequently, we hypothesize that M2 macrophages could be concerned with a key regulator of peritoneal dissemination in gastric cancer.
In the current study, we examined intraperitoneal macrophages from ascites or peritoneal lavage samples in gastric cancer patients, and evaluated their phenotype (M1 and M2). In addition, we assessed whether M2 macrophages contribute to tumor malignant phenotypes in gastric cancer.
Materials and methods
Patient samples
Ascites or peritoneal lavage samples were collected from 11 gastric cancer patients with peritoneal dissemination. At the time of surgery, 50 ml of ascites or 100 ml of peritoneal lavage sample was collected. Control samples (ten patients) were obtained from patients with benign tumors (gastrointestinal stromal tumor) or patients with stage I gastric cancer. The clinicopathological characteristics of the patients are presented in Table 1. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all patients according to the protocols approved by the Kanazawa University Hospital Review Board (study number 1463).
Fluorescence-activated cell sorting
Samples were centrifuged at 1500 rpm for 5 min, then washed with fluorescence-activated cell sorting buffer (phosphate-buffered saline plus 2 % fetal bovine serum). The samples were filtered through a 100-μm mesh for flow cytometry. The cells were subsequently stained with the following surface markers for 30 min at 4 °C: phycoerythrin (PE)–Cy7-labeled anti-human CD45 (BD Biosciences, San Diego, CA, USA); PE-labeled anti-human CD163 (BD Biosciences); PE-labeled anti-human CD204 (R&D Systems, Minneapolis, MN, USA); and PerCP–Cy5.5-labeled anti-human CCR2 (BioLegend, San Diego, CA, USA). Intracellular staining (30 min, 4 °C) was performed with fluorescein isothiocyanate labeled anti-human CD68 (BD Biosciences) after permeabilization for 20 min at 4 °C with a BD Cytofix/Cytoperm Plus fixation/permeabilization kit (BD Biosciences). As negative controls, isotype control antibodies (Biolegend) were used. Cells were analyzed by flow cytometry by means of an Attune acoustic cytometer (Applied Biosystems; Life Technologies, Carlsbad, CA, USA). Data were transferred and reanalyzed with FlowJo (Tree Star, Oregon, OR, USA).
Quantitative real-time reverse transcription polymerase chain reaction
Total RNA was extracted from the macrophages with use of RNeasy mini kits (Qiagen, Germantown, MD, USA) and was treated with an RNase-free DNase set (Qiagen). The integrity of isolated RNA was verified by analytical agarose gel electrophoresis. First-strand complementary DNA was prepared from 2-µg aliquots of DNase-treated RNA with use of complementary DNA synthesis kits. The primers for tumor necrosis factor α (TNF-α), CD80, CD86, interleukin (IL)-12p40, IL-10, vascular endothelial growth factor (VEGF)-A, VEGF-C, matrix metalloproteinase (MMP)-1, epidermal growth factor (EGF), amphiregulin, and TATA-binding protein (TBP) were designed with Primer Express. The primers used for this analysis are shown in Table 2. The expression of each of the primers was normalized relative to that of TBP in the same sample. The polymerase chain reaction (PCR) mixtures for TNF-α, CD80, CD86, IL-12p40, IL-10, VEGF-A, VEGF-C, MMP-1, EGF, amphiregulin, and TBP contained 13 SYBR Green Master Mix (Life Technologies), complementary DNA template, and optimized primer concentrations, diluted to a final volume of 25 ml with nuclease-free water. All PCRs were performed with an ABI Prism 7900 sequence detection system (Applied Biosystems, Foster City, CA, USA).
Cell lines and cell culture
Monocytes were isolated from healthy human donors. Peripheral blood was collected from healthy volunteers in conical tubes containing 0.5 ml heparin and mixed with an equal volume of saline. Peripheral blood mononuclear cells (PBMCs) were isolated with LymphoPrep tubes (Axis-Shield, Dundee, UK) following the manufacturer’s protocol and cultured in RPMI-1640 medium supplemented with 10 % fetal bovine serum, penicillin, and streptomycin. After 24 h the nonadherent cells were removed by gentle aspiration, and the medium was replaced. We confirmed that more than 90 % of adherent cells were CD14+ by flow cytometry. Monocytes were then differentiated into M1 macrophages by exposure to granulocyte-macrophage colony stimulating factor (50 ng/ml; Wako, Tokyo, Japan) for at most 5 days, and were treated with lipopolysaccharide (100 ng/ml; Wako) and interferon-γ (20 ng/ml; Peprotech, Rocky Hill, NJ, USA) for 24 h [22]. To induce M2 macrophages, monocytes were cultured with monocyte colony stimulating factor (100 ng/ml; Wako) for at most 5 days, and were treated with IL-4 (20 ng/ml; Peprotech), IL-10 (20 ng/ml; MACS, Miltenyi Biotec, Bergisch Gladbach, Germany), and IL-13 (20 ng/ml; R&D Systems) for 24 h [22].
Human gastric cancer cell lines, TMK-1 (poorly differentiated adenocarcinoma) and MKN45 (poorly differentiated adenocarcinoma), were obtained from American Type Culture Collection (Rockville, MD, USA). TMK-1 and MKN45 cells were cultured in RPMI-1640 medium supplemented with 10 % fetal bovine serum, penicillin, and streptomycin. The cells were seeded in gelatin-coated 75-cm2 flasks (BD BioCoat, Boston, MA, USA) and cultured in 10 ml of medium at 37 °C in a humidified atmosphere of 5 % CO2 in air. We routinely checked Mycoplasma infection, and negative results were obtained with a PCR Mycoplasma test kit (Promokine, Heidelberg, Germany).
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay buffer [50 mmol/l tris(hydroxymethyl)aminomethane–HCl (pH 8.0), 150 mmol/l sodium chloride, 0.5 w/v % sodium deoxycholate, 0.1 w/v % sodium dodecyl sulfate, 1.0 w/v % NP-40 substitute (Wako)] containing 1 % protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA) and 1 % phosphatase inhibitor (Sigma-Aldrich); the protein concentration of each lysate was measured with a bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL, USA). Whole cell lysates were prepared in denaturing sodium dodecyl sulfate sample buffer and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Bio-Rad, Philadelphia, PA, USA). Proteins were transferred to poly(vinylidene fluoride) membranes (Bio-Rad) and blocked with commercial gradient buffer (EzBlock, Atto) at room temperature for 30 min. Membranes were incubated with the following primary antibodies: anti-phosphorylated signal transducer and activator of transcription 3 (STAT3; rabbit monoclonal IgG, diluted 1:1000; Cell Signaling Technology, Danvers, MA, USA), anti-CD163 (mouse monoclonal IgG, diluted 1:200; Santa Cruz Biotechnology), anti-phosphorylated EGF receptor (EGFR; rabbit monoclonal IgG, diluted 1:1000; Cell Signaling Technology), anti-phosphorylated AKT (rabbit monoclonal IgG, diluted 1:1000; Cell Signaling Technology), anti-phosphorylated extracellular-signal-regulated kinase 1/2 (ERK1/2; rabbit monoclonal IgG, diluted 1:1000; Cell Signaling Technology), and anti-β-actin (mouse monoclonal IgG, diluted 1:10,000; Sigma-Aldrich). After incubation with secondary antibodies, the antibody–antigen complexes were detected with an ECL Western blotting detection kit (GE Healthcare Japan) and a LightCapture system (Atto).
Cell proliferation assay
MKN45 cells seeded at a density of 1 × 105 cells per well in six-well plates were incubated alone (control) or in the presence of a direct co-culture, with the same number of M2 macrophages. A 1-μm pore Boyden chamber (BD Falcon) was used for indirect incubation. Cells were counted on days 1, 2, and 3 after seeding. After CD326 expression had been confirmed only in MKN45 cells, not in M2 macrophages, by flow cytometry, the magnetic-activated cell sorting (Miltenyi Biotec) method with microbead-labeled anti-human CD326 antibody (Miltenyi Biotec) was applied to separate MKN45 cells from M2 macrophages.
Quantification of cytokine levels
Levels of amphiregulin and heparin-binding EGF-like growth factor (HB-EGF) secreted in culture medium were quantified by use of specific ELISA system kits (R&D Systems).
Mouse xenograft model
Animals were treated in accordance with the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions, under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology of Japan. All animal experiments were approved by the Committee on Animal Experimentation of Kanazawa University. We used BALB/c nu/nu mice (females aged 4–6 weeks; Charles River Laboratories, Japan) as a xenograft model to see the effects of co-inoculation of MKN45 cells and M2 macrophages. The cells were injected subcutaneously into the dorsal side of the mouse (n = 12). Into each of the six control mice, 7 × 106 MKN45 cells in 100 μl of phosphate-buffered saline were injected, whereas we designed the cell implantation with the same total number of 5 × 106 MKN45 cells plus 2 × 106 M2 macrophages in 100 μl of phosphate-buffered saline in the experimental group (n = 6). Xenograft tumor size was measured every other day for 14 days. Tumor volume was estimated with the equation V = (ab 2)/2, where V is volume, a is the length of the major axis, and b is the length of the minor axis. After 14 days the mice were killed and the tumors were removed for immunohistochemical examination.
Immunohistochemistry
Tumor specimens were fixed in 10 % neutral buffered formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin and immunostained with anti-Ki67 antibody (Santa Cruz Biotechnology) and anti-CD31 antibody (abcam, San Francisco, CA, USA). Deparaffinized sections were pretreated by autoclaving in 10 % citric acid buffer (pH 8.0) at 120 °C for 15 min. Following treatment with protein block serum (Dako Cytomation, Kyoto, Japan) for 10 min and incubation with 2 % skim milk for 30 min to block nonspecific reactions, sections were incubated with primary antibody at 4 °C overnight. The EnVision polymer solution (horseradish peroxidase; Dako Cytomation) was then applied for 1 h. Signals were developed in 0.02 % 3,3′-diaminobenzidinetetrahydrochloride solution containing 0.1 %. As negative controls, the sections were incubated with tris(hydroxymethyl)aminomethane-buffered saline with non-immune mouse IgG (Santa Cruz Biotechnology) or non-immune rabbit IgG (Santa Cruz Biotechnology). Sections were then lightly counterstained with hematoxylin and examined under a fluorescence microscope (Olympus, Tokyo, Japan). For endothelial cell counting in tumors, five vascularized areas in a visual field (×100) were chosen, and the number of CD31+ cells was then counted.
Statistical analysis
We investigated differences among the data sets by one-way analysis of variance or two-sided Student’s t tests with the computer software package SPSS 10.0 (SPSS, Chicago, IL, USA). P values less than 0.05 indicated a statistically significant difference.
Results
Intraperitoneal macrophages from gastric cancer patients with peritoneal dissemination were polarized to the M2 phenotype
Figure 1a shows photomicrographs obtained using phase contrast microscopy of M1 macrophages, M2 macrophages and TAMs from a gastric cancer patient with peritoneal dissemination. PBMC-derived M1 macrophages exhibited classical round macrophage morphology, in contrast to the bipolar appearance of PBMC-derived M2 macrophages (Fig. 1a). TAMs exhibited a bipolar appearance similar to PBMC-derived M2 macrophages (Fig. 1a).
Intraperitoneal macrophage phenotype in gastric cancer patients with peritoneal dissemination a Phase contrast microscopy of M1 and M2 macrophages differentiated from peripheral blood mononuclear cells and tumor-associated macrophages (TAM) from a gastric cancer patient with peritoneal dissemination. Magnification ×400. b Intraperitoneal cells were collected from peritoneal lavage samples or ascites with or without peritoneal dissemination, and analyzed by flow cytometry. The fluorescence-activated cell sorting profiles for a representative case are shown. In patients with peritoneal dissemination, a large number of CD45+CD68+ macrophages were present in the peritoneal cavity. c The characteristics of intraperitoneal macrophages in gastric cancer with or without peritoneal dissemination were determined by flow cytometry. The ratios of macrophages positive for CD68 in CD45+ cells (CD68+/CD45+), CD163 in CD68+ cells (CD163+/CD68+), CD204 in CD68+ cells (CD204+/CD68+), and CCR2 in CD68+ cells (CCR2+/CD68+) were evaluated in gastric cancer patients with or without peritoneal dissemination. Values represent the mean ± standard error (n = 21)
We also characterized the surface markers of the peritoneal macrophages in gastric cancer patients with or without peritoneal dissemination by flow cytometry. Figure 1b shows the large number of cells positive for CD45 (pan-leukocyte marker) that existed in the peritoneal cavity of a patient with peritoneal dissemination. The ratio of macrophages positive for CD68 (pan-macrophage marker) in CD45+ cells (CD68+/CD45+) in gastric cancer patients with peritoneal dissemination was significantly higher than that in patients without peritoneal dissemination (Fig. 1c). Moreover, the ratio of CD163+ cells (M2 macrophage marker) in CD68+ cells (CD163+/CD68+), CD204+ cells (M2 macrophage marker) in CD68+ cells (CD204+/CD68+), or CCR2+ cells in CD68+ cells (CCR2+/CD68+) in patients with peritoneal dissemination was significantly higher than that in patients without peritoneal dissemination (P < 0.01; Fig. 1c). These data indicate that macrophages abundantly accumulated with invaded gastric cancer cells in the abdominal cavity of patients with peritoneal dissemination and largely tended to be of the M2 phenotype.
Gene expression profile of gastric cancer TAMs
Gene expression profiles of TAMs in various cancers have been reported [23, 24]. With reference to the gene expression pattern, we performed reverse transcription PCR to compare gastric cancer TAMs with peritoneal dissemination and representative M1 macrophages derived from PBMCs in terms of expression levels of messenger RNAs (mRNAs), which could be associated with tumor progression or suppression. As a result, the levels of M1-related gene expression of TNF-α, CD80, CD86, and IL-12p40 were significantly lower in the TAMs relative to the M1 macrophages (Fig. 2). In contrast, M2-related gene expression levels such as for IL-10, VEGF-A, VEGF-C, MMP-1, and amphiregulin were significantly increased in the TAMs (Fig. 2). We could not find any significant differences in mRNA levels of EGF, HB-EGF, MMP-2, MMP-9, MMP-14, CCL17, CCL18, CCL22, and TGF-β between the two (data not shown).
Gene expression profile of gastric cancer tumor-associated macrophages and peripheral blood mononuclear cell (PBMC)-derived M1 macrophages obtained by reverse transcription polymerase chain reaction. The M1-related messenger RNAs (TNF-α, CD80, CD86, and IL-12p40) and M2-related messenger RNAs [IL-10, vascular endothelial growth factor A (VEGF-A), vascular endothelial growth factor C (VEGF-C), matrix metalloproteinase 1 (MMP-1), and amphiregulin] were measured in tumor-associated macrophages from gastric cancer patients with peritoneal dissemination and PBMC-derived M1 macrophages. Values represent the mean ± standard error (n = 14). mRNA messenger RNA
Co-culture of gastric cancer cells switched M1 macrophages to the M2 phenotype
Tumor-derived factors such as IL-4, IL10, and monocyte colony stimulating factor are reported to cause TAMs to polarize toward the M2 phenotype [15, 25, 26]. Therefore, we next examined in vitro effects of MKN45 or TMK-1 gastric cancer cells on macrophage characteristics by use of co-culture systems. As shown in Fig. 3, M2 marker CD163 expression was found to be upregulated in PBMC-derived M1 macrophages after they had been co-cultured with MKN45 or TMK-1 cells for 24 or 48 h. In addition, the level of phosphorylated STAT3, which is a key molecule for the M2 phenotype, was also dramatically increased by co-existence of MKN45 or TMK-1 cells (Fig. 3).
Increase of gastric cancer cell proliferation by co-culture with M2 macrophages
There may be some communication between peritoneal disseminated gastric cancer cells and the M2 macrophages accumulated in the peritoneal cavity, leading to the formation of a tumor microenvironment. We next investigated phenotypic changes in tumor cells by co-culture with M2 macrophages. The MKN45 cell number was elevated after co-culture with M2 macrophages, and this increase was significantly higher in the case of a direct co-cultured system than in the case of an indirect one (Fig. 4a).
a Gastric cancer cell proliferation effects of a direct and an indirect co-culture of MKN45 cells with or without M2 macrophages. The MKN45 cell count was elevated, and cell count ratio is shown. Values represent the mean ± standard error (n = 3). b Western blot analyses of phosphorylated epidermal growth factor receptor (pEGFR), phosphorylated AKT (pAKT), phosphorylated extracellular-signal-regulated kinase 1/2 (pERK1/2), and phosphorylated signal transducer and activator of transcription 3 (pSTAT3). Levels of phosphorylation of epidermal growth factor receptor, AKT, extracellular-signal-regulated kinase 1/2, and signal transducer and activator of transcription 3 were increased in MKN45 cells after they had been co-cultured with M2 macrophages. c Amphiregulin levels in gastric cancer cells and M1 and M2 macrophages measured by an ELISA system. Values represent the mean ± standard error (n = 3)
Increased expression of phosphorylation of EGFR, AKT, ERK1/2, and STAT3 in MKN45 cells caused by M2 macrophages
We next investigated cell-growth-related factors in MKN45 cells after co-culture with M2 macrophages. Figure 4b shows time-dependent increases of expression in phosphorylation of EGFR, AKT, ERK1/2, and STAT3 in MKN45 cells after co-culture with M2 macrophages. We then tried to identify the main regulator derived from TAMs with the M2 phenotype, which could affect the cancer cell biology. For that, we focused on EGFR, which is known to be associated with the progression of various tumor types [27, 28]. Moreover, the levels of its ligands amphiregulin and HB-EGF are reported to be markedly higher in malignant ascites in gastric cancer than in nonmalignant peritoneal exudates [29]. We thus measured the levels of amphiregulin and HB-EGF in both the macrophages and MKN45 cells. HB-EGF was found to be under the detection level in the macrophages and MKN45 cells when we used an ELISA (data not shown). The level of amphiregulin in M2 macrophages was detectable and significantly higher than that in M1 macrophages (Fig. 4c). Moreover, the level of amphiregulin in M2 macrophages with MKN45 cells was significantly higher than that in M2 macrophages alone (Fig. 4c). A similar tendency was observed in M1 macrophages with MKN45 cells (Fig. 4c), suggesting that this might be caused by switching of macrophages from the M1 to the M2 phenotype and the resultant increase of amphiregulin expression in macrophages.
In vivo effect of M2 macrophages on tumor growth in a xenograft model
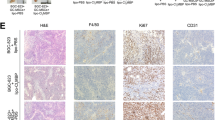
To see in vivo effects of TAMs on tumor phenotype, we used a mouse xenograft model. MKN45 cells alone at 7.0 × 106 cells or 5.0 × 106 MKN45 cells plus 2.0 × 106 M2 macrophages were subcutaneously injected into the dorsal side of BALB/c nu/nu mice. The xenografted tumor size and volume were then evaluated, and the data obtained are shown in Fig. 5a. We confirmed that PBMC-derived M2 macrophages (CD163+) existed in the xenograft tissue (Fig. 5c). At 15 days after inoculation, the mean volume of the tumor grown from MKN45 cells and M2 macrophages was significantly greater than that grown from MKN45 cells alone (P < 0.01). Representative results for the tumors are shown in Fig. 5b, and immunohistochemical stainings with Ki-67 showed higher cell proliferation in the co-inoculated group when compared with the control group inoculated with MKN45 cells alone (Fig. 5d; P < 0.05). In addition, immunohistochemical staining with anti-mouse CD31 antibody was conducted to investigate the microvessel density. CD31+ endothelial cell number was found to be significantly higher in the co-inoculated group than in the control group (Fig. 5e), indicating that angiogenesis was enhanced in the tumor by the coexistence of M2 macrophages.
a In vivo effect of M2 macrophages on tumor growth in a xenograft model. a The mean volume of the tumors co-inoculated with MKN45 and M2 macrophages was evaluated until 15 days after inoculation. Values represent the mean ± standard error (n = 6). b Representative images showing the macroscopic appearance of the tumors at day 15. c M2 macrophages derived from peripheral blood mononuclear cells (anti-human CD163 antibody) were found in xenograft tissue. d Immunohistochemical staining with Ki-67 in the co-inoculated group and the control group inoculated with MKN45 cells alone. e Immunohistochemical staining with anti-mouse CD31 antibody. Microvessel density was evaluated as CD31+ endothelial cell number per visual field (×100)
Discussion
The tumor microenvironment in the peritoneal cavity consists of many different types of cells, including peritoneal mesothelial cells, macrophages, endothelial cells, fibroblasts, and lymphocytes. Liu et al. [30] reported that milky spot macrophages remodeled to the M2 phenotype by gastric cancer cells promoted human peritoneal mesothelial cell injury, which contributed to the formation of peritoneal dissemination. However, it is unknown if macrophages contribute to the development of peritoneal dissemination in gastric cancer. In this study, we first investigated the phenotype of intraperitoneal macrophages in gastric cancer. A high number of macrophages were observed in patients with peritoneal dissemination, and most had the M2 phenotype (Fig. 1c). In addition, most macrophages in the peritoneal cavity were chemokine receptor type 2 (CCR2)-positive cells (Fig. 1c). The CC chemokine ligand CCL2 induces monocytes to migrate from the bloodstream and enter the surrounding tissue to become tissue macrophages [31]. CCR2-positive monocytes could be preferentially recruited to CCL2 produced by cancer cells. It is reported that this CCL2–CCR2 axis can polarize macrophages to an alternatively activated M2 phenotype, thereby contributing to immunosuppression and enhanced tumor survival [32, 33]. Therefore, it is reasonable that macrophages are recruited to the peritoneal cavity of patients with peritoneal dissemination. Furthermore, we found that M1 macrophages were induced to differentiate into the M2 phenotype by coexistence of gastric cancer cells (Fig. 3). Cancer-cell-derived factors, including IL-6, IL-10, TGF-β, and monocyte colony stimulating factor, are believed to induce macrophages to polarize to the M2 phenotype [25]; TAMs are recruited from the circulating monocytes that are attracted by tumor-derived chemokines and are polarized to M2-resembling macrophages by growth factors and cytokines in the tumor microenvironment.
A hallmark of macrophages is their plasticity. Depending on external stimuli, macrophages can acquire different phenotypes with partly opposing properties. From the reverse transcription PCR data regarding macrophages in the peritoneal cavity, M1-related mRNAs such as those of TNF-α, CD80, CD86, and IL-12p40 were less strongly expressed in TAMs relative to PBMC-derived M1 macrophages (Fig. 2). In contrast, M2-related mRNAs such as those of IL-10, VEGF-A, VEGF-C, MMP-1, and amphiregulin were more strongly expressed in TAMs. (Figure 2). It is well known that VEGF plays a major role in angiogenesis, and is associated with the development of peritoneal metastasis and malignant ascites [34]. IL-10 produced by TAMs is an immunosuppressive cytokine, contributing to the general suppression of anti-tumor activities in the tumor environment [25]. MMP expression has been implicated in tumor invasion and metastasis [35]. These data suggest that the soluble factors derived from TAMs are responsible for the tumor activity in the microenvironment, and stimulate tumor proliferation and progression in peritoneal dissemination. No significant differences were found in the mRNA levels of EGF, HB-EGF, MMP-2, MMP-9, MMP-14, CCL17, CCL18, CCL22, and TGF-β between TAMs and PBMC-derived macrophages (data not shown). In our study, most TAMs from patients with peritoneal dissemination had the M2 macrophage markers (CD163 and CD204) (Fig. 1c). However, this classification is a purely operational definition, because macrophages can adopt a large variety of phenotypes deviating from this classification, and may even acquire the properties of both M1 and M2 cells [23, 36, 37].
We found that the proliferation of gastric cancer cells in direct contact with M2 macrophages was significantly higher than in the indirect co-culture system (Fig. 4a). This finding indicates that not only macrophage-derived soluble factors but also direct cell-to-cell contact can contribute to malignant phenotypes of cancer cells. Komohara et al. [19] reported that direct cell-to-cell contact between macrophages and cancer cells induced significant differentiation of macrophages to the M2 phenotype with the expression of CD163 and the production of IL-10. In addition, direct co-culture with M2 macrophages significantly stimulated STAT3 activation in cancer cells, resulting in cancer cell proliferation and progression [19]. In vivo, subcutaneous inoculation of MKN45 cells with M2 macrophages resulted in significantly larger tumors than was the case for MKN45 cells alone (Fig. 5a). The Ki-67 index and microvessel density were also higher in the co-inoculated group than in the control group inoculated with MKN45 cells alone, suggesting that the coexistence of M2 macrophages could play a role in gastric cancer progression.
Previous studies have reported that a number of cytokines, chemokines, and growth factors from macrophages promote tumor progression. In our study, the mRNA levels of the EGFR ligand, amphiregulin, were higher in TAMs from patients with peritoneal dissemination (Fig. 2). In addition, the level of phosphorylated EGFR in MKN45 cells was enhanced after co-culture with M2 macrophages (Fig. 4b). We also confirmed that amphiregulin was produced more significantly by M2 macrophages in vitro (Fig. 4c). These results indicate that the EGFR–amphiregulin axis is important in the development of peritoneal dissemination in gastric cancer. Amphiregulin receptor EGFR overexpression is likely to be an independent indicator of poor prognosis in gastric cancer [27, 38, 39]. It was reported that EGFR was detected in 27.4–44.0 % of gastric cancer samples in several studies [27, 38–41]. In contrast, overexpression of EGFR in gastric cancer with peritoneal dissemination was 79 % [29]. Therefore, intracellular signaling pathways of EGFR might relate to the formation and development of peritoneal dissemination. EGFR is activated by binding to its ligands, resulting in homodimerization or heterodimerization with another member of the EGFR family [42]. This receptor activation is followed by phosphorylation of specific tyrosine residues, including AKT, ERK1/2, and STAT3, stimulating the downstream signaling pathway that regulates cell proliferation, migration, adhesion, differentiation, and survival. In this study, we found enhanced expression of phosphorylation of AKT, ERK1/2, and STAT3 in gastric cancer cells (Fig. 4b).
In conclusion, TAMs in the peritoneal cavity of gastric cancer patients with peritoneal dissemination were polarized to the M2 phenotype, and M2 macrophages contributed to the development of peritoneal dissemination via activation of the EGFR signaling pathways. Consequently, intraperitoneal TAMs are expected to become a target for treatment of peritoneal dissemination in gastric cancer.
References
Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–43.
Fushida S, Kinoshita J, Yagi Y, Funaki H, Kinami S, Ninomiya I, et al. Dual anti-cancer effects of weekly intraperitoneal docetaxel in treatment of advanced gastric cancer patients with peritoneal carcinomatosis: a feasibility and pharmacokinetic study. Oncol Rep. 2008;19:1305–10.
Fushida S, Kinoshita J, Kaji M, Hirono Y, Goda F, Yagi Y, et al. Phase I/II study of intraperitoneal docetaxel plus S-1 for the gastric cancer patients with peritoneal carcinomatosis. Cancer Chemother Pharmacol. 2013;71:1265–72.
Shimada S, Tanaka E, Marutsuka T, Honmyo U, Tokunaga H, Yagi Y, et al. Extensive intraoperative peritoneal lavage and chemotherapy for gastric cancer patients with peritoneal free cancer cells. Gastric Cancer. 2002;5:168–72.
Yonemura Y, Kawamura T, Nojima N, Bandou E, Keizou T, Fujita H, et al. Postoperative results of left upper abdominal evisceration for advanced gastric cancer. Hepatogastroenterology. 2010;47:571–4.
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21.
Shirao K, Boku N, Yamada Y, Yamaguchi K, Doi T, Goto M, et al. Randomized phase III study of 5-fluorouracil continuous infusion vs sequential methotrexate and 5-fluorouracil therapy in far advanced gastric cancer with peritoneal metastasis (JCOG0106). Jpn J Clin Oncol. 2013;43:972–80.
Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401.
Tlsty TD. Stromal cells can contribute oncogenic signals. Semin Cancer Biol. 2001;11:97–104.
Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–8.
Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–31.
Tsukada T, Fushida S, Harada S, Yagi Y, Kinoshita J, Oyama K, et al. The role of human peritoneal mesothelial cells in the fibrosis and progression of gastric cancer. Int J Oncol. 2012;41:476–82.
Terai S, Fushida S, Tsukada T, Kinoshita J, Oyama K, Okamoto K, et al. Bone marrow derived “fibrocytes” contribute to tumor proliferation and fibrosis in gastric cancer. Gastric Cancer. 2015;18:306–13.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7.
Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12.
Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216:15–24.
Niino D, Komohara Y, Murayama T, Aoki R, Kimura Y, Hashikawa K, et al. Ratio of M2 macrophage expression is closely associated with poor prognosis for angioimmunoblastic T-cell lymphoma (AITL). Pathol Int. 2010;60:278–83.
Hasita H, Komohara Y, Okabe H, Masuda T, Ohnishi K, Lei XF, et al. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101:1913–9.
Komohara Y, Hasita H, Ohnishi K, Fujiwara Y, Suzu S, Eto M, et al. Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci. 2011;102:1424–31.
Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Okumura H, Matsumoto M, et al. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res. 2003;23:4079–83.
Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer. 2014;134:32–42.
Jaguin M, Houlbert N, Fardel O, Lecureul V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol. 2013;281:51–61.
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51.
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55.
Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarized population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–27.
Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65.
Galizia G, Lieto E, Orditura M, Castellano P, Mura AL, Imperatore V, et al. Epidermal growth factor receptor (EGFR) expression is associated with a worse prognosis in gastric cancer patients undergoing curative surgery. World J Surg. 2007;31:1458–68.
Galizia G, Lieto E, Ferraraccio F, Vita De, Castellano P, Orditura M, et al. Prognositic significance of epidermal growth factor receptor expression in colon cancer patients undergoing curative surgery. Ann Surg Oncol. 2006;13:823–35.
Yasumoto K, Yamada T, Kawashima A, Wang W, Li Q, Donev IS, et al. The EGFR ligands amphiregulin and heparin-binding egf-like growth factor promote peritoneal carcinomatosis in CXCR4-expressing gastric cancer. Clin Cancer Res. 2011;1:3619–30.
Liu XY, Miao ZF, Zhao TT, Wang ZN, Xu YY, Gao J, et al. Milky spot macrophages remodeled by gastric cancer cells promote peritoneal mesothelial cell injury. Biochem Biophys Res Commun. 2013;439:378–83.
Wyler L, Napoli CU, Ingold B, Sulser T, Heikenwälder M, Schrami P, et al. Brain metastasis in renal cancer patients: metastatic pattern, tumour-associated macrophages and chemokine/chemoreceptor expression. Br J Cancer. 2014;110:686–94.
Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007;67:9417–24.
Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7.
Fushida S, Oyama K, Kinoshita J, Yagi Y, Okamoto K, Tajima H, et al. VEGF is a target molecule for peritoneal metastasis and malignant ascites in gastric cancer: prognostic significance of VEGF in ascites and efficacy of anti-VEGF monoclonal antibody. Onco Targets Ther. 2013;6:1445–51.
Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunol Lett. 2009;123:97–102.
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68.
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95.
Lieto E, Ferraraccio F, Orditura M, Castellano P, Mura AL, Pinto M, et al. Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol. 2008;15:69–79.
Anzai H, Kitadai Y, Bucana CD, Sanchez R, Omoto R, Fidler IJ. Expression of metastasis-related genes in surgical specimens of human gastric cancer can predict disease recurrence. Eur J Cancer. 1998;34:558–65.
Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH, et al. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology. 2008;52:738–46.
Weng X, Zhang H, Ye J, Kan M, Liu F, Wang T, et al. Hypermethylated Epidermal growth factor receptor (EGFR) promoter is associated with gastric cancer. Sci Rep. 2015;11:10154.
Brown CL, Meise KS, Plowman GD, Coffey RJ, Dempsey PJ. Cell surface ectodomain cleavage of human amphiregulin precursor is sensitive to a metalloprotease inhibitor. Release of a predominant N-glycosylated 43-kDa soluble form. J Biol Chem. 1998;273:17258–68.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for their being included in the study (study number 1463).
Animal studies
All institutional and national guidelines for the care and use of laboratory animals were followed. Animals were treated in accordance with the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions, under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology of Japan. All animal experiments were approved by the Committee on Animal Experimentation of Kanazawa University.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit (http://creativecommons.org/licenses/by-nc-nd/4.0/)
About this article
Cite this article
Yamaguchi, T., Fushida, S., Yamamoto, Y. et al. Tumor-associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer 19, 1052–1065 (2016). https://doi.org/10.1007/s10120-015-0579-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-015-0579-8