Abstract
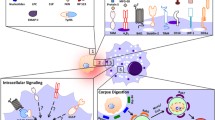
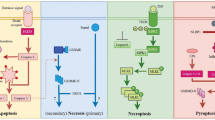
The ultimate and most favorable fate of almost all dying cells is engulfment by neighboring or specialized cells. Efficient clearance of cells undergoing apoptotic death is crucial for normal tissue homeostasis and for the modulation of immune responses. Engulfment of apoptotic cells is finely regulated by a highly redundant system of receptors and bridging molecules on phagocytic cells that detect molecules specific for dying cells. Recognition of necrotic cells by phagocytes is less well understood than recognition of apoptotic cells, but an increasing number of recent studies, which are discussed here, are highlighting its importance. New observations indicate that the interaction of macrophages with dying cells initiates internalization of the apoptotic or necrotic targets, and that internalization can be preceded by “zipper”-like and macropinocytotic mechanisms, respectively. We emphasize that clearance of dying cells is an important fundamental process serving multiple functions in the regulation of normal tissue turnover and homeostasis, and is not just simple anti- or pro-inflammatory responses. Here we review recent findings on genetic pathways participating in apoptotic cell clearance, mechanisms of internalization, and molecules involved in engulfment of apoptotic versus necrotic cells, as well as their immunological consequences and relationships to disease pathogenesis.
Similar content being viewed by others
References
Baehrecke EH (2002) How death shapes life during development. Nat Rev Mol Cell Biol 3:779–787
Ellis RE, Jacobson DM, Horvitz HR (1991) Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics 129:79–94
Gumienny TL, Hengartner MO (2001) How the worm removes corpses: the nematode C. elegans as a model system to study engulfment. Cell Death Differ 8:564–568
Reddien PW, Horvitz HR (2004) The engulfment process of programmed cell death in caenorhabditis elegans. Annu Rev Cell Dev Biol 20:193–221
de Bakker CD, Haney LB, Kinchen JM, et al (2004) Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr Biol 14:2208–2216
Henson PM (2005) Engulfment: ingestion and migration with Rac, Rho and TRIO. Curr Biol 15:R29–30
Zhou Z, Hartwieg E, Horvitz HR (2001) CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell 104:43–56
Su HP, Nakada-Tsukui K, Tosello-Trampont AC, et al (2002) Interaction of CED-6/GULP, an adapter protein involved in engulfment of apoptotic cells with CED-1 and CD91/low density lipoprotein receptor-related protein (LRP). J Biol Chem 277:11772–11779
Wu YC, Tsai MC, Cheng LC, Chou CJ, Weng NY (2001) C. elegans CED-12 acts in the conserved crkII/DOCK180/Rac pathway to control cell migration and cell corpse engulfment. Dev Cell 1:491–502
Wu YC, Horvitz HR (1998) The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell 93:951–960
Liu QA, Hengartner MO (1998) Candidate adaptor protein CED-6 promotes the engulfment of apoptotic cells in C. elegans. Cell 93:961–972
Kinchen JM, Cabello J, Klingele D, et al (2005) Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature 434:93–99
Abrams JM, White K, Fessler LI, Steller H (1993) Programmed cell death during Drosophila embryogenesis. Development 117:29–43
Franc NC (2002) Phagocytosis of apoptotic cells in mammals, caenorhabditis elegans and Drosophila melanogaster:molecular mechanisms and physiological consequences. Front Biosci 7:d1298–1313
Franc NC, Heitzler P, Ezekowitz RA, White K (1999) Requirement for croquemort in phagocytosis of apoptotic cells in Drosophila. Science 284:1991–1994
Manaka J, Kuraishi T, Shiratsuchi A, et al (2004) Draper-mediated and phosphatidylserine-independent phagocytosis of apoptotic cells by Drosophila hemocytes/macrophages. J Biol Chem 279:48466–48476
Emoto K, Toyama-Sorimachi N, Karasuyama H, Inoue K, Umeda M (1997) Exposure of phosphatidylethanolamine on the surface of apoptotic cells. Exp Cell Res 232:430–434
Fadeel B (2003) Programmed cell clearance. Cell Mol Life Sci 60:2575–2585
Grimsley C, Ravichandran KS (2003) Cues for apoptotic cell engulfment: eat-me, don’t eat-me and come-get-me signals. Trends Cell Biol 13:648–656
Arur S, Uche UE, Rezaul K, et al (2003) Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell 4:587–598
de Almeida CJ, Linden R (2005) Phagocytosis of apoptotic cells: a matter of balance. Cell Mol Life Sci 62:1532–1546
Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 148:2207–2216
Schlegel RA, Williamson P (2001) Phosphatidylserine, a death knell. Cell Death Differ 8:551–563
Fadok VA, Bratton DL, Frasch SC, Warner ML, Henson PM (1998) The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ 5:551–562
Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL (2001) Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem 276:1071–1077
Williamson P, Schlegel RA (2002) Transbilayer phospholipid movement and the clearance of apoptotic cells. Biochim Biophys Acta 1585:53–63
Hamon Y, Broccardo C, Chambenoit O, et al (2000) ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat Cell Biol 2:399–406
Kagan VE, Gleiss B, Tyurina YY, et al (2002) A role for oxidative stress in apoptosis: oxidation and externalization of phosphatidylserine is required for macrophage clearance of cells undergoing Fas-mediated apoptosis. J Immunol 169:487–499
Dillon SR, Mancini M, Rosen A, Schlissel MS (2000) Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation. J Immunol 164:1322–1332
Dillon SR, Constantinescu A, Schlissel MS (2001) Annexin V binds to positively selected B cells. J Immunol 166:58–71
Kuijpers TW, Maianski NA, Tool AT, et al (2004) Neutrophils in Barth syndrome (BTHS) avidly bind annexin-V in the absence of apoptosis. Blood 103:3915–3923
Wanderley JL, Benjamin A, Real F, Bonomo A, Moreira ME, Barcinski MA (2005) Apoptotic mimicry:an altruistic behavior in host/Leishmania interplay. Braz J Med Biol Res 38:807–812
Wanderley JL, Moreira ME, Benjamin A, Bonomo AC, Barcinski MA (2006) Mimicry of apoptotic cells by exposing phosphatidylserine participates in the establishment of amastigotes of Leishmania (L) amazonensis in mammalian hosts. J Immunol 176:1834–1839
Elliott JI, Surprenant A, Marelli-Berg FM, et al (2005) Membrane phosphatidylserine distribution as a non-apoptotic signalling mechanism in lymphocytes. Nat Cell Biol 7:808–816
Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J (2002) Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature 418:200–203
Gardai SJ, McPhillips KA, Frasch SC, et al (2005) Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123:321–334
Borisenko GG, Matsura T, Liu SX, et al (2003) Macrophage recognition of externalized phosphatidylserine and phagocytosis of apoptotic Jurkat cells–existence of a threshold. Arch Biochem Biophys 413:41–52
Balasubramanian K, Schroit AJ (2003) Aminophospholipid asymmetry: a matter of life and death. Annu Rev Physiol 65:701–734
Appelt U, Sheriff A, Gaipl US, Kalden JR, Voll RE, Herrmann M (2005) Viable, apoptotic and necrotic monocytes expose phosphatidylserine: cooperative binding of the ligand Annexin V to dying but not viable cells and implications for PS-dependent clearance. Cell Death Differ 12:194–196
Platt N, da Silva RP, Gordon S (1998) Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol 8:365–372
Fadok VA, Warner ML, Bratton DL, Henson PM (1998) CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3). J Immunol 161:6250–6257
Mevorach D, Mascarenhas JO, Gershov D, Elkon KB (1998) Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med 188:2313–2320
Fadok VA, Henson PM (2003) Apoptosis:giving phosphatidylserine recognition an assist–with a twist. Curr Biol 13:R655–R657
Balasubramanian K, Schroit AJ (1998) Characterization of phosphatidylserine-dependent beta2-glycoprotein I macrophage interactions. Implications for apoptotic cell clearance by phagocytes. J Biol Chem 273:29272–29277
Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417:182–187
Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu H, Shacter E (2003) Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat Immunol 4:87–91
Chen J, Carey K, Godowski PJ (1997) Identification of Gas6 as a ligand for Mer, a neural cell adhesion molecule related receptor tyrosine kinase implicated in cellular transformation. Oncogene 14:2033–2039
Savill J, Hogg N, Ren Y, Haslett C (1992) Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest 90:1513–1522
Borisenko GG, Iverson SL, Ahlberg S, Kagan VE, Fadeel B (2004) Milk fat globule epidermal growth factor 8 (MFG-E8) binds to oxidized phosphatidylserine:implications for macrophage clearance of apoptotic cells. Cell Death Differ 11:943–945
Lecoeur H, Prevost MC, Gougeon ML (2001) Oncosis is associated with exposure of phosphatidylserine residues on the outside layer of the plasma membrane:a reconsideration of the specificity of the annexin V/propidium iodide assay. Cytometry 44:65–72
Krysko O, De Ridder L, Cornelissen M (2004) Phosphatidylserine exposure during early primary necrosis (oncosis) in JB6 cells as evidenced by immunogold labeling technique. Apoptosis 9:495–500
Hirt UA, Leist M (2003) Rapid, noninflammatory and PS-dependent phagocytic clearance of necrotic cells. Cell Death Differ 10:1156–1164
Brouckaert G, Kalai M, Krysko DV, et al (2004) Phagocytosis of necrotic cells by macrophages is phosphatidylserine dependent and does not induce inflammatory cytokine production. Mol Biol Cell 15:1089–1100
Bottcher A, Gaipl US, Furnrohr BG, et al (2006) Involvement of phosphatidylserine, alphavbeta3, CD14, CD36, and complement C1q in the phagocytosis of primary necrotic lymphocytes by macrophages. Arthritis Rheum 54:927–938
Cocco RE, Ucker DS (2001) Distinct modes of macrophage recognition for apoptotic and necrotic cells are not specified exclusively by phosphatidylserine exposure. Mol Biol Cell 12:919–930
Nauta AJ, Trouw LA, Daha MR, et al (2002) Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur J Immunol 32:1726–1736
Nauta AJ, Raaschou-Jensen N, Roos A, et al (2003) Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol 33:2853–2863
Rovere P, Peri G, Fazzini F, et al (2000) The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood 96:4300–4306
Roos A, Xu W, Castellano G, et al (2004) Mini-review: a pivotal role for innate immunity in the clearance of apoptotic cells. Eur J Immunol 34:921–929
Zwart B, Ciurana C, Rensink I, Manoe R, Hack CE, Aarden LA (2004) Complement activation by apoptotic cells occurs predominantly via IgM and is limited to late apoptotic (secondary necrotic) cells. Autoimmunity 37:95–102
Gaipl US, Kuenkele S, Voll RE, et al (2001) Complement binding is an early feature of necrotic and a rather late event during apoptotic cell death. Cell Death Differ 8:327–334
Ciurana CL, Zwart B, van Mierlo G, Hack CE (2004) Complement activation by necrotic cells in normal plasma environment compares to that by late apoptotic cells and involves predominantly IgM. Eur J Immunol 34:2609–2619
Hart SP, Alexander KM, MacCall SM, Dransfield I (2005) C-reactive protein does not opsonize early apoptotic human neutrophils, but binds only membrane-permeable late apoptotic cells and has no effect on their phagocytosis by macrophages. J Inflamm (Lond) 2:5
Hart SP, Ross JA, Ross K, Haslett C, Dransfield I (2000) Molecular characterization of the surface of apoptotic neutrophils:implications for functional downregulation and recognition by phagocytes. Cell Death Differ 7:493–503
Jones AL, Poon IK, Hulett MD, Parish CR (2005) Histidine-rich glycoprotein specifically binds to necrotic cells via its amino-terminal domain and facilitates necrotic cell phagocytosis. J Biol Chem 280:35733–35741
Fujii C, Shiratsuchi A, Manaka J, Yonehara S, Nakanishi Y (2001) Difference in the way of macrophage recognition of target cells depending on their apoptotic states. Cell Death Differ 8:1113–1122
Nakai Y, Shiratsuchi A, Manaka J, et al (2005) Externalization and recognition by macrophages of large subunit of eukaryotic translation initiation factor 3 in apoptotic cells. Exp Cell Res 309:137–148
Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM (2000) A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405:85–90
Hong JR, Lin GH, Lin CJ, et al (2004) Phosphatidylserine receptor is required for the engulfment of dead apoptotic cells and for normal embryonic development in zebrafish. Development 131:5417–5427
Wang X, Wu YC, Fadok VA, et al (2003) Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12. Science 302:1563–1566
Li MO, Sarkisian MR, Mehal WZ, Rakic P, Flavell RA (2003) Phosphatidylserine receptor is required for clearance of apoptotic cells. Science 302:1560–1563
Kunisaki Y, Masuko S, Noda M, et al (2004) Defective fetal liver erythropoiesis and T lymphopoiesis in mice lacking the phosphatidylserine receptor. Blood 103:3362–3364
Bose J, Gruber AD, Helming L, et al (2004) The phosphatidylserine receptor has essential functions during embryogenesis but not in apoptotic cell removal. J Biol 3:15
Hanayama R, Tanaka M, Miyasaka K, et al (2004) Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 304:1147–1150
Cohen PL, Caricchio R, Abraham V, et al (2002) Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med 196:135–140
Mitchell JE, Cvetanovic M, Tibrewal N, et al (2006) The Presumptive Phosphatidylserine Receptor Is Dispensable for Innate Anti-inflammatory Recognition and Clearance of Apoptotic Cells. J Biol Chem 281:5718–5725
Albert ML, Kim JI, Birge RB (2000) Alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol 2:899–905
Cui P, Qin B, Liu N, Pan G, Pei D (2004) Nuclear localization of the phosphatidylserine receptor protein via multiple nuclear localization signals. Exp Cell Res 293:154–163
Cikala M, Alexandrova O, David CN, et al (2004) The phosphatidylserine receptor from Hydra is a nuclear protein with potential Fe(II) dependent oxygenase activity. BMC Cell Biol 5:26
Clissold PM, Ponting CP (2001) JmjC: cupin metalloenzyme-like domains in jumonji, hairless and phospholipase A2beta. Trends Biochem Sci 26:7–9
Kurushima H, Ramprasad M, Kondratenko N, Foster DM, Quehenberger O, Steinberg D (2000) Surface expression and rapid internalization of macrosialin (mouse CD68) on elicited mouse peritoneal macrophages. J Leukoc Biol 67:104–108
Swanson JA, Watts C (1995) Macropinocytosis. Trends Cell Biol 5:424–428
Swanson JA, Baer SC (1995) Phagocytosis by zippers and triggers. Trends Cell Biol 5:89–93
Griffin FM, Jr, Griffin JA, Leider JE, Silverstein SC (1975) Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med 142:1263–1282
Griffin FM, Jr, Griffin JA, Silverstein SC (1976) Studies on the mechanism of phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG-coated bone marrow-derived lymphocytes. J Exp Med 144:788–809
Kaplan G, Bertheussen K (1977) The morphology of echinoid phagocytes and mouse peritoneal macrophages during phagocytosis in vitro. Scand J Immunol 6:1289–1296
Allen LA, Aderem A (1996) Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J Exp Med 184:627–637
Caron E, Hall A (1998) Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282:1717–1721
Giles KM, Hart SP, Haslett C, Rossi AG, Dransfield I (2000) An appetite for apoptotic cells? Controversies and challenges. Br J Haematol 109:1–12
Alpuche-Aranda CM, Racoosin EL, Swanson JA, Miller SI (1994) Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J Exp Med 179:601–608
Torii I, Morikawa S, Nagasaki M, Nokano A, Morikawa K (2001) Differential endocytotic characteristics of a novel human B/DC cell line HBM-Noda: effective macropinocytic and phagocytic function rather than scavenging function. Immunology 103:70–80
Rittig MG, Burmester GR, Krause A (1998) Coiling phagocytosis: when the zipper jams, the cup is deformed. Trends Microbiol 6:384–388
Rittig MG, Wilske B, Krause A (1999) Phagocytosis of microorganisms by means of overshooting pseudopods: where do we stand? Microbes Infect 1:727–735
Krysko DV, Brouckaert G, Kalai M, Vandenabeele P, D’Herde K (2003) Mechanisms of internalization of apoptotic and necrotic L929 cells by a macrophage cell line studied by electron microscopy. J Morphol 258:336–345
Krysko DV, Denecker G, Festjens N, et al (2006) Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ
Ogden CA, de Cathelineau A, Hoffmann PR, et al (2001) C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med 194:781–795
Hoffmann PR, de Cathelineau AM, Ogden CA, et al (2001) Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol 155:649–659
Fiorentini C, Falzano L, Fabbri A, et al (2001) Activation of rho GTPases by cytotoxic necrotizing factor 1 induces macropinocytosis and scavenging activity in epithelial cells. Mol Biol Cell 12:2061–2073
Jersmann HP, Dransfield I, Hart SP (2003) Fetuin/alpha2-HS glycoprotein enhances phagocytosis of apoptotic cells and macropinocytosis by human macrophages. Clin Sci (Lond) 105:273–278
Xu W, Roos A, Schlagwein N, Woltman AM, Daha MR, van Kooten C (2006) IL-10-producing macrophages preferentially clear early apoptotic cells. Blood
Chung S, Gumienny TL, Hengartner MO, Driscoll M (2000) A common set of engulfment genes mediates removal of both apoptotic and necrotic cell corpses in C. elegans. Nat Cell Biol 2:931–937
Cardelli J (2001) Phagocytosis and macropinocytosis in Dictyostelium: phosphoinositide-based processes, biochemically distinct. Traffic 2:311–320
Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101:890–898
Huynh ML, Fadok VA, Henson PM (2002) Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest 109:41–50
Xiao YQ, Malcolm K, Worthen GS, et al (2002) Cross-talk between ERK and p38 MAPK mediates selective suppression of pro-inflammatory cytokines by transforming growth factor-beta. J Biol Chem 277:14884–14893
Cvetanovic M, Ucker DS (2004) Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J Immunol 172:880–889
Kim S, Elkon KB, Ma X (2004) Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity 21:643–653
Gao Y, Herndon JM, Zhang H, Griffith TS, Ferguson TA (1998) Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J Exp Med 188:887–896
Chen W, Frank ME, Jin W, Wahl SM (2001) TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity 14:715–725
Byrne A, Reen DJ (2002) Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J Immunol 168:1968–1977
Savill J, Dransfield I, Gregory C, Haslett C (2002) A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2:965–975
Ren Y, Stuart L, Lindberg FP, et al (2001) Nonphlogistic clearance of late apoptotic neutrophils by macrophages: efficient phagocytosis independent of beta 2 integrins. J Immunol 166:4743–4750
Patel VA, Longacre A, Hsiao K, et al (2006) Apoptotic Cells, at All Stages of the Death Process, Trigger Characteristic Signaling Events That Are Divergent from and Dominant over Those Triggered by Necrotic Cells: Implications For The Delayed Clearance Model Of Autoimmunity. J Biol Chem 281:4663–4670
Devitt A, Parker KG, Ogden CA, et al (2004) Persistence of apoptotic cells without autoimmune disease or inflammation in CD14–/–mice. J Cell Biol 167:1161–1170
Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA (2005) Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol 174:3220–3226
Fadok VA, Bratton DL, Guthrie L, Henson PM (2001) Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol 166:6847–6854
Zheng L, He M, Long M, Blomgran R, Stendahl O (2004) Pathogen-induced apoptotic neutrophils express heat shock proteins and elicit activation of human macrophages. J Immunol 173:6319–6326
Lorimore SA, Coates PJ, Scobie GE, Milne G, Wright EG (2001) Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects? Oncogene 20:7085–7095
Hart SP, Alexander KM, Dransfield I (2004) Immune complexes bind preferentially to Fc gamma RIIA (CD32) on apoptotic neutrophils, leading to augmented phagocytosis by macrophages and release of proinflammatory cytokines. J Immunol 172:1882–1887
Gregory CD, Devitt A (2004) The macrophage and the apoptotic cell: an innate immune interaction viewed simplistically? Immunology 113:1–14
Mitchell DA, Pickering MC, Warren J, et al (2002) C1q deficiency and autoimmunity: the effects of genetic background on disease expression. J Immunol 168:2538–2543
Chautan M, Chazal G, Cecconi F, Gruss P, Golstein P (1999) Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr Biol 9:967–970
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
Bianchi ME, Manfredi A (2004) Chromatin and cell death. Biochim Biophys Acta 1677:181–186
Park JS, Svetkauskaite D, He Q, et al (2004) Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279:7370–7377
Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK (2000) Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 12:1539–1546
Shi Y, Evans JE, Rock KL (2003) Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425:516–521
Binder RJ, Vatner R, Srivastava P (2004) The heat-shock protein receptors: some answers and more questions. Tissue Antigens 64:442–451
Tsan MF (2006) Toll-like receptors, inflammation and cancer. Semin Cancer Biol 16:32–37
Kariko K, Ni H, Capodici J, Lamphier M, Weissman D (2004) mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem 279:12542–12550
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732–738
Ishii KJ, Suzuki K, Coban C, et al (2001) Genomic DNA released by dying cells induces the maturation of APCs. J Immunol 167:2602–2607
Krysko DV, Leybaert L, Vandenabeele P, D’Herde K (2005) Gap junctions and the propagation of cell survival and cell death signals. Apoptosis 10:459–469
Skoberne M, Beignon AS, Bhardwaj N (2004) Danger signals: a time and space continuum. Trends Mol Med 10:251–257
Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, Robaye B (2001) The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol 166:7172–7177
Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K (1997) Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. J Cell Biol 139:1635–1643
Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR (2005) Potentiation of caspase-1 activation by the P27 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol 175:7611–7622
Vanden Berghe T, Kalai M, Denecker G, Meeus A, Saelens X, Vandenabeele P (2006) Necrosis is associated with IL-6 production but apoptosis is not. Cell Signal 18:328–335
Saelens X, Festjens N, Parthoens E, et al (2005) Protein synthesis persists during necrotic cell death. J Cell Biol 168:545–551
Saelens X, Kalai M, Vandenabeele P (2001) Translation inhibition in apoptosis: caspase-dependent PKR activation and eIF2-alpha phosphorylation. J Biol Chem 276:41620–41628
Clemens MJ, Bushell M, Jeffrey IW, Pain VM, Morley SJ (2000) Translation initiation factor modifications and the regulation of protein synthesis in apoptotic cells. Cell Death Differ 7:603–615
Reddy SM, Hsiao KH, Abernethy VE, et al (2002) Phagocytosis of apoptotic cells by macrophages induces novel signaling events leading to cytokine-independent survival and inhibition of proliferation: activation of Akt and inhibition of extracellular signal-regulated kinases 1 and 2. J Immunol 169:702–713
Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K (2005) Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22:285–294
Ryoo HD, Gorenc T, Steller H (2004) Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell 7:491–501
Golpon HA, Fadok VA, Taraseviciene-Stewart L, et al (2004) Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. Faseb J 18:1716–1718
Ware LB, Matthay MA (2002) Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol 282:L924–L940
Morimoto K, Amano H, Sonoda F, et al (2001) Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am J Respir Cell Mol Biol 24:608–615
Stefansson S, McMahon GA, Petitclerc E, Lawrence DA (2003) Plasminogen activator inhibitor-1 in tumor growth, angiogenesis and vascular remodeling. Curr Pharm Des 9:1545–1564
Salani D, Taraboletti G, Rosano L, et al (2000) Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol 157:1703–1711
Salani D, Di Castro V, Nicotra MR, et al (2000) Role of endothelin-1 in neovascularization of ovarian carcinoma. Am J Pathol 157:1537–1547
Ramanathan M, Giladi A, Leibovich SJ (2003) Regulation of vascular endothelial growth factor gene expression in murine macrophages by nitric oxide and hypoxia. Exp Biol Med (Maywood) 228:697–705
Li M, Carpio DF, Zheng Y, et al (2001) An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol 166:7128–7135
Shinohara K, Shinohara T, Mochizuki N, et al (1996) Expression of vascular endothelial growth factor in human myocardial infarction. Heart Vessels 11:113–122
Sharkey AM, Charnock-Jones DS, Boocock CA, Brown KD, Smith SK (1993) Expression of mRNA for vascular endothelial growth factor in human placenta. J Reprod Fertil 99:609–615
Shweiki D, Itin A, Soffer D, Keshet E (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843–845
Lang RA, Bishop JM (1993) Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell 74:453–462
Diez-Roux G, Argilla M, Makarenkova H, Ko K, Lang RA (1999) Macrophages kill capillary cells in G1 phase of the cell cycle during programmed vascular regression. Development 126:2141–2147
Duffield JS, Erwig LP, Wei X, Liew FY, Rees AJ, Savill JS (2000) Activated macrophages direct apoptosis and suppress mitosis of mesangial cells. J Immunol 164:2110–2119
Reddien PW, Cameron S, Horvitz HR (2001) Phagocytosis promotes programmed cell death in C. elegans. Nature 412:198–202
Hoeppner DJ, Hengartner MO, Schnabel R (2001) Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature 412:202–206
Brown SB, Savill J (1999) Phagocytosis triggers macrophage release of Fas ligand and induces apoptosis of bystander leukocytes. J Immunol 162:480–485
Trinite B, Chauvin C, Peche H, Voisine C, Heslan M, Josien R (2005) Immature CD4- CD103+ rat dendritic cells induce rapid caspase-independent apoptosis-like cell death in various tumor and nontumor cells and phagocytose their victims. J Immunol 175:2408–2417
Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N (2000) Consequences of cell death:exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 191:423–434
Wong OH, Huang FP, Chiang AK (2005) Differential responses of cord and adult blood-derived dendritic cells to dying cells. Immunology 116:13–20
Kacani L, Wurm M, Schwentner I, Andrle J, Schennach H, Sprinzl GM (2005) Maturation of dendritic cells in the presence of living, apoptotic and necrotic tumour cells derived from squamous cell carcinoma of head and neck. Oral Oncol 41:17–24
Barker RN, Erwig LP, Hill KS, Devine A, Pearce WP, Rees AJ (2002) Antigen presentation by macrophages is enhanced by the uptake of necrotic, but not apoptotic, cells. Clin Exp Immunol 127:220–225
Chen Q, Stone PR, McCowan LM, Chamley LW (2006) Phagocytosis of necrotic but not apoptotic trophoblasts induces endothelial cell activation. Hypertension 47:116–121
Scheffer SR, Nave H, Korangy F, et al (2003) Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int J Cancer 103:205–211
Goldszmid RS, Idoyaga J, Bravo AI, Steinman R, Mordoh J, Wainstok R (2003) Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma. J Immunol 171:5940–5947
Casares N, Pequignot MO, Tesniere A, et al (2005) Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 202:1691–1701
Janssens S, Tinel A, Lippens S, Tschopp J (2005) PIDD mediates NF-kappaB activation in response to DNA damage. Cell 123:1079–1092
Lillehei KO, Liu Y, Kong Q (1999) Current perspectives in immunotherapy. Ann Thorac Surg 68:S28–S33
Leist M, Jaattela M (2001) Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2:589–598
Bondanza A, Zimmermann VS, Rovere-Querini P, et al (2004) Inhibition of phosphatidylserine recognition heightens the immunogenicity of irradiated lymphoma cells in vivo. J Exp Med 200:1157–1165
Ran S, Thorpe PE (2002) Phosphatidylserine is a marker of tumor vasculature and a potential target for cancer imaging and therapy. Int J Radiat Oncol Biol Phys 54:1479–1484
Ran S, Downes A, Thorpe PE (2002) Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res 62:6132–6140
Ran S, He J, Huang X, Soares M, Scothorn D, Thorpe PE (2005) Antitumor effects of a monoclonal antibody that binds anionic phospholipids on the surface of tumor blood vessels in mice. Clin Cancer Res 11:1551–1562
Huang X, Bennett M, Thorpe PE (2005) A monoclonal antibody that binds anionic phospholipids on tumor blood vessels enhances the antitumor effect of docetaxel on human breast tumors in mice. Cancer Res 65:4408–4416
Beck AW, Luster TA, Miller AF, et al (2006) Combination of a monoclonal anti-phosphatidylserine antibody with gemcitabine strongly inhibits the growth and metastasis of orthotopic pancreatic tumors in mice. Int J Cancer 118:2639–2643
Hart SP, Smith JR, Dransfield I (2004) Phagocytosis of opsonized apoptotic cells: roles for ‘old-fashioned’ receptors for antibody and complement. Clin Exp Immunol 135:181–185
Fadeel B, Orrenius S, Pervaiz S (2004) Buried alive: a novel approach to cancer treatment. Faseb J 18:1–4
Ren Y, Tang J, Mok MY, Chan AW, Wu A, Lau CS (2003) Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum 48:2888–2897
Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR (1998) Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum 41:1241–1250
Munoz LE, Gaipl US, Franz S, et al (2005) SLE-a disease of clearance deficiency? Rheumatology (Oxford) 44:1101–1107
Vandivier RW, Fadok VA, Hoffmann PR, et al (2002) Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest 109:661–670
Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M (2003) Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol 81:289–296
O’Brien BA, Fieldus WE, Field CJ, Finegood DT (2002) Clearance of apoptotic beta-cells is reduced in neonatal autoimmune diabetes-prone rats. Cell Death Differ 9:457–464
Baumann I, Kolowos W, Voll RE, et al (2002) Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum 46:191–201
Berden JH (2003) Lupus nephritis:consequence of disturbed removal of apoptotic cells? Neth J Med 61:233–238
Casciola-Rosen LA, Anhalt GJ, Rosen A (1995) DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J Exp Med 182:1625–1634
Casiano CA, Martin SJ, Green DR, Tan EM (1996) Selective cleavage of nuclear autoantigens during CD95 (Fas/APO-1)-mediated T cell apoptosis. J Exp Med 184:765–770
Donnelly S, Roake W, Brown S, et al (2006) Impaired recognition of apoptotic neutrophils by the C1q/calreticulin and CD91 pathway in systemic lupus erythematosus. Arthritis Rheum 54:1543–1556
Asano K, Miwa M, Miwa K, et al (2004) Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J Exp Med 200:459–467
Author information
Authors and Affiliations
Corresponding author
Additional information
Katharina D’Herde and Peter Vandenabeele share senior authorship.
This study was supported by Ghent University GOA grant No. 12050502, IUAP-V/12-12.0C14.02, FWO-Vlaanderen 3G.0218.06, and Flanders Interuniversity Institute for Biotechnology (VIB).
Rights and permissions
About this article
Cite this article
Krysko, D.V., D’Herde, K. & Vandenabeele, P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis 11, 1709–1726 (2006). https://doi.org/10.1007/s10495-006-9527-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-9527-8