Abstract
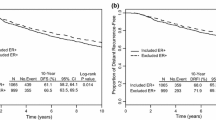
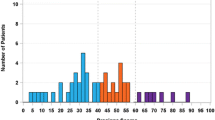
The Oncotype DX® Recurrence Score® (RS) is a validated genomic predictor of outcome and response to adjuvant chemotherapy in ER-positive breast cancer. Adjuvant! was developed using SEER registry data and results from the Early Breast Cancer Clinical Trialists’ overview analyses to estimate outcome and benefit from adjuvant hormonal therapy and chemotherapy. In this report we compare the prognostic and predictive utility of these two tools in node-negative, ER-positive breast cancer. RS and Adjuvant! results were available from 668 tamoxifen-treated NSABP B-14 patients, 227 tamoxifen-treated NSABP B-20 patients, and 424 chemotherapy plus tamoxifen-treated B-20 patients. Adjuvant! results were also available from 1952 B-20 patients. The primary endpoint was distant recurrence-free interval (DRFI). Cox proportional hazards models were used to compare the prognostic and predictive utility of RS and Adjuvant!. Both RS (P < 0.001) and Adjuvant! (P = 0.002) provided strong independent prognostic information in tamoxifen-treated patients. Combining RS and individual clinicopathologic characteristics provided greater prognostic discrimination than combining RS and the composite Adjuvant!. In the B-20 cohort with RS results (n = 651), RS was significantly predictive of chemotherapy benefit (interaction P = 0.031 for DRFI, P = 0.011 for overall survival [OS], P = 0.082 for disease-free survival [DFS]), but Adjuvant! was not (interaction P = 0.99, P = 0.311, and P = 0.357, respectively). However, in the larger B-20 sub-cohort (n = 1952), Adjuvant! was significantly predictive of chemotherapy benefit for OS (interaction P = 0.009) but not for DRFI (P = 0.219) or DFS (P = 0.099). Prognostic estimates can be optimized by combining RS and clinicopathologic information instead of simply combining RS and Adjuvant!. RS should be used for estimating relative chemotherapy benefit.





Similar content being viewed by others
References
Sorlie T, Perou CM, Tibshirani R et al (2004) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. PNAS 98:10869–10874
Van De Vijver M, He YD, Van’t Veer LJ et al (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999–2009
Van’t Veer LJ, Dai H, van de Vijver MJ et al (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530–536
Foekens JA, Atkins D, Zhang Y et al (2006) Multicenter validation of a gene expression-based prognostic signature in lymph node-negative primary breast cancer. J Clin Oncol 24:1665–1671
Paik S, Shak S, Tang G et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826
Dowsett M, Cuzick J, Wales C et al (2009) Risk of distant recurrence using oncotype DX in postmenopausal primary breast cancer patients treated with anastrozole or tamoxifen: a TransATAC study. Cancer 69:75 abstr 53
Habel LA, Shak S, Jacobs MK et al (2006) A population-based study of tumor gene expression, risk of breast cancer death among lymph node-negative patients. Breast Cancer Res 8:R25
Paik S, Tang G, Shak S et al (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726–3734
Albain KS, Barlow WE, Shak S et al (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11:55–65
Harris L, Fritsche H, Mennel R et al (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25:5287–5312
NCCN Clinical Practice Guidelines in OncologyTM Breast Cancer, (Version 2.2008). http://www.nccn.org. Accessed 18 Sept 2008
Ravdin PM, Siminoff LA, Davis GJ et al (2001) Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol 19:980–991
Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 17 Regs Limited Use + Katrina Impacted Louisiana Cases. Nov Released April 2008, based on the November 2007 submission. Surveillance Research Program, National Cancer Institute SEER* Software. [version 6.4.4]. www.seer.cancer.gov/seerstat. Accessed 29 Jan 2010
“The Early Breast Cancer Clinical Trialist’ Collaborative Group” (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
Olivotto IA, Bajdik CD, Ravdin PM et al (2005) Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol 23:2716–2725
Goldstein LJ, Gray R, Badve S et al (2008) Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol 26:4063–4071
Dowsett M, Cuzick J, Wale C et al (2010) Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 28:1829–1834
Fisher B, Costantino J, Redmond C et al (1989) A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med 320:479–484
Fisher B, Dignam J, Bryant J et al (2001) Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst 93:684–690
Fisher B, Dignam J, Wolmark N et al (1997) Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst 89:1673–1682
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Assersohn L, Salter J, Powles TJ et al (2003) Studies of the potential utility of Ki67 as a predictive molecular marker of clinical response in primary breast cancer. Breast Cancer Res Treat 82:113–123
Burcombe RJ, Makris A, Richman PI et al (2005) Evaluation of ER, PgR, HER-2 and Ki-67 as predictors of response to neoadjuvant anthracycline chemotherapy for operable breast cancer. Br J Cancer 92:147–155
Cheang MCU, Chia SK, Voduc D et al (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101:736–750
Penault-Llorca F, Andre F, Sagan C et al (2009) Ki67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer. J Clin Oncol 27:2809–2815
Acknowledgments
This study is supported in part by Public Health Service Grants U10CA-12027, U10CA-69974, U10CA-37377, U10CA-69651, and U24-CA-114732 from the National Cancer Institute, Department of Health and Human Services. The funding source did not affect the study design, data collection, analysis, interpretation, writing, or submission of this work. We would like to thank Dr. Drew Watson, Genomic Health, for valuable discussions during this study.
Conflicts of interest
Even though the NSABP Statistical Center has received research funding from Genomic Health, this study was not supported by Genomic Health. SS is a full-time employee and a stockholder in Genomic Health. GT, SJA, and JPC declare no potential conflicts of interest. EPM has been a consultant and on the speaker’s bureau of Genomic Health. There are no other potential conflicts of interest reported.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, G., Shak, S., Paik, S. et al. Comparison of the prognostic and predictive utilities of the 21-gene Recurrence Score assay and Adjuvant! for women with node-negative, ER-positive breast cancer: results from NSABP B-14 and NSABP B-20. Breast Cancer Res Treat 127, 133–142 (2011). https://doi.org/10.1007/s10549-010-1331-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1331-z