Abstract
To identify a group of patients who might benefit from the addition of weekly paclitaxel to conventional anthracycline-containing chemotherapy as adjuvant therapy of node-positive operable breast cancer. The predictive value of PAM50 subtypes and the 11-gene proliferation score contained within the PAM50 assay were evaluated in 820 patients from the GEICAM/9906 randomized phase III trial comparing adjuvant FEC to FEC followed by weekly paclitaxel (FEC-P). Multivariable Cox regression analyses of the secondary endpoint of overall survival (OS) were performed to determine the significance of the interaction between treatment and the (1) PAM50 subtypes, (2) PAM50 proliferation score, and (3) clinical and pathological variables. Similar OS analyses were performed in 222 patients treated with weekly paclitaxel versus paclitaxel every 3 weeks in the CALGB/9342 and 9840 metastatic clinical trials. In GEICAM/9906, with a median follow up of 8.7 years, OS of the FEC-P arm was significantly superior compared to the FEC arm (unadjusted HR = 0.693, p = 0.013). A benefit from paclitaxel was only observed in the group of patients with a low PAM50 proliferation score (unadjusted HR = 0.23, p < 0.001; and interaction test, p = 0.006). No significant interactions between treatment and the PAM50 subtypes or the various clinical–pathological variables, including Ki-67 and histologic grade, were identified. Finally, similar OS results were obtained in the CALGB data set, although the interaction test did not reach statistical significance (p = 0.109). The PAM50 proliferation score identifies a subset of patients with a low proliferation status that may derive a larger benefit from weekly paclitaxel.
Similar content being viewed by others
Introduction
Administration of taxanes in the adjuvant setting improves disease-free survival and overall survival (OS) in early breast cancer, although the absolute survival benefit is rather small across studies (~3–7 %) [1–4]. To date, taxanes are beneficial in the adjuvant setting irrespective of the patient’s age, lymph-node involvement, estrogen-receptor status, and HER2 status [1–4]. Weekly paclitaxel and every-3 week docetaxel seem to be the most effective means of administering these drugs in the adjuvant setting [5]. However, taxanes are associated with toxic side-effects and, thus, identification of which patients benefit (or not) from the addition of this class of drugs is an unmet medical need.
Over the last decade, global gene expression profiling has given us insights into the biological complexity of breast tumors, and clinically applicable gene expression-based assays are being developed for the prediction of prognosis and/or treatment benefit [6–14]. Among them, the PAM50 classifier identifies the four major biologic subtypes of breast cancer referred to as Luminal A, Luminal B, HER2-enriched, and Basal-like. The genomic subtype classification has shown clinical application for prognosis in patients receiving only surgical treatment without adjuvant therapy [11], and adjuvant endocrine blockade without chemotherapy [14]. In this study, we explored the possible interaction between paclitaxel treatment and overall survival (OS) with (1) the PAM50 intrinsic subtypes, (2) the PAM50 proliferation score, and (3) various clinical–pathological variables using tissue blocks collected from the phase III GEICAM/9906 trial. A subsequent similar analysis for OS was also performed in a second data set of breast cancer patients with metastatic disease from the CALGB/9342 and 9840 trials, which compared weekly paclitaxel versus paclitaxel every 3 weeks.
Materials and methods
Patients, samples and clinical data
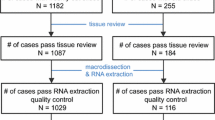
The GEICAM/9906 trial was a prospective adjuvant multi-center randomized phase III study (n = 1,246 subjects) comparing six cycles of FEC (control arm) versus four cycles of FEC followed by eight weekly cycles of paclitaxel at 100 mg/m2 (FEC-P, experimental arm) in node-positive breast cancers in the era before adjuvant trastuzumab was available. Hormonal therapy followed chemotherapy in patients with estrogen and/or progesterone receptor positive disease. The primary endpoint of the GEICAM/9906 clinical trial was disease-free survival. Secondary endpoints were: (a) OS; (b) prognostic and predictive value of molecular/genomic markers; and (c) safety. The study was performed in accordance with the Declaration of Helsinki, approved by the ethics committees at all participating institutions and the Spanish Health Authority, and it was registered at www.clinicaltrials.gov (identifier code: NCT00129922). All patients provided written informed consent for therapy randomization and molecular analyses. Details of the study design and patients’ characteristics have been previously reported [15, 16]. Formalin-fixed, paraffin-embedded (FFPE) tumor blocks were available on 825 patients. H&E sections from each FFPE tissue block were reviewed by a pathologist at GEICAM’s central laboratory. At least two tumor cores were extracted from areas containing representative invasive breast carcinoma using a 1 mm core punch. A detailed protocol of RNA extraction from FFPE tissue and the RT-qPCR PAM50 assay have been previously described [11].
PAM50 subtype classification
Samples were gene expression profiled using the previously described RT-qPCR assay and analyzed using the clinical algorithm for subtype prediction [11, 17] (online resource Fig. S1). Samples were assigned into the following intrinsic subtype categories: Luminal A, Luminal B, HER2-enriched, Basal-like, and Normal-like. Samples classified as Normal-like were excluded from further analyses due to the potential for misclassification resulting from normal breast tissue or stroma contamination within the tumor specimen [18]. In addition to the subtype classification, we calculated a PAM50 proliferation score using the previously described 11-gene signature (BIRC5, CCNB1, CDC20, CDCA1, CEP55, KNTC2, MKI67, PTTG1, RRM2, TYMS, UBE2C) [14]. The significance of proliferation was evaluated using a classification into quartiles, and using the proliferation scores as a continuous variable.
Immunohistochemical (IHC) Ki-67 quantification
Ki-67 status was assessed in a central laboratory on paraffin sections by an immunohistochemical method using Clone MIB 1 antibody (DakoCytomation, Glostrup, Denmark). Ki67 score was defined as the percentage of total number of tumor cells with nuclear staining.
Independent evaluation using the CALGB/9342 and 9840 data set
The CALGB/9342 clinical trial compared three different doses (175, 210, and 250 mg/m2) of paclitaxel administered every 3 weeks, and the higher doses did not improve response rate (primary endpoint) or overall survival (secondary endpoint) [19]. The phase III CALGB/9840 clinical trial showed superiority of weekly paclitaxel versus paclitaxel every 3 weeks in metastatic breast cancer based on an increased response rate (primary endpoint) [20]. Samples from 240 patients of the CALGB/9342 and 9840 trials had FFPE tumor tissue blocks available for nucleic acid/RNA extraction. Invasive disease was identified on H&E sections, and one to three 1.5 mm cores were punched from the top down in the designated tumor areas of each FFPE block. The cores were deparaffinized with xylene at 50 °C for 3 min. RNA was extracted using the RecoverAll Total Nucleic Acid Isolation kit (Applied Biosystems) following the manufacturer’s protocol. Tumor samples from 237 patients had adequate RNA for hybridization. The isolated RNA was hybridized to Whole-Genome DASL (HumanRef8 V 3.0, Illumina) at the Yale Center for Genome Analysis. Of 1,048 patients included in the CALGB/9342 and 9840 trials, 222 FFPE individual primary tumors were successfully gene expression profiled using the DASL platform (online resource Fig. S2). Patient characteristics of the combined data set can be found in online resource Table S1.
Statistical analysis
The analysis of the GEICAM/9906 trial has a prospective–retrospective design (retrospective analysis of a randomized prospective trial) with pre-specified study objectives and pre-specified laboratory assays in a predefined population [21]. The primary pre-specified objectives of the current study were to determine whether the PAM50 subtypes, and/or the PAM50 proliferation score, were associated with OS and/or predictive of paclitaxel benefit. The Kaplan–Meier method was used to estimate overall survival (OS), and the log-rank test was used to compare OS between groups. Univariate and multivariate Cox proportional hazard models were used to examine the association of each variable with survival and interaction between treatment and PAM50 subtype and proliferation. Similar OS analyses were performed in the CALGB/9342 and 9840 combined data set. The results are presented in accordance with reporting recommendations for tumor marker prognostic studies (REMARK) criteria [22].
Results
Patient demographics
Tumor blocks were available for 829 patients from the GEICAM/9906 trial, and PAM50 genomic profiling was successful in 820 samples (99.4 %) of patients whose informed consent was obtained (online resource Fig. S1), which represents 66 % of the original 1,246 sample set of the GEICAM/9906 trial [15]. The demographic and prognostic features, as well as the 8-year OS of patients included in this sub-study were similar to those of the overall study population (data not shown). The distribution of the patient’s clinical–pathological characteristics included in this study is shown in Table 1.
Overall survival outcomes
In the study population (N = 820), with a median follow up of 8.7 years, OS of the FEC-P arm was significantly superior compared to the FEC arm (unadjusted hazard ratio [HR] for OS 0.693, 95 % confidence interval [CI] 0.519–0.927, p = 0.013) with 8-year OS rates in the FEC and FEC-P arms of 76 and 82 %, respectively (Fig. 1a). These results are consistent with OS analysis in GEICAM/9906 trial. Univariate analysis revealed the following variables significantly associated with OS: menopausal status, nodal status, histopathologic grade, tumor size, ER status, PR status, Ki67, PAM50 subtypes, and PAM50 proliferation score. As expected, the PAM50 Luminal A tumors showed the best outcome (88 % OS at 8 years), followed by Luminal B (76 %), Basal-like (70 %), and HER2-enriched (71 %) (Fig. 1b). Compared to Luminal A tumors, Luminal B, HER2-enriched, and Basal-like tumors showed an unadjusted HR for OS of 1.99 (1.35–2.97), 2.60 (1.52–4.40), and 2.62 (1.74–3.95), respectively. Interestingly, although the PAM50 proliferation score and Ki-67 were found significantly associated with OS (Fig. 2), the separation of the curves by quartile distribution was found to be greater using the PAM50 proliferation score compared to Ki-67 by IHC.
Among the variables evaluated, tumor size, nodal status, and PAM50 proliferation score were found to be independent predictors of OS in multivariate analysis with treatment arm showing a tendency for significance (p = 0.067) (online resource Table S2). Of note, Ki-67 by IHC and histologic grade were superseded by the information provided by the PAM50 proliferation score.
Effect of paclitaxel in the PAM50 subtypes and by proliferation score
Kaplan–Meier plots for OS comparing treatment with FEC-P versus FEC were evaluated in each group category defined by the PAM50 assay. The individual PAM50 subtypes were not found to be predictive of paclitaxel efficacy. On the other hand, a benefit from paclitaxel was observed in patients whose tumors had a low PAM50 proliferation score (unadjusted HR = 0.23 within the lowest quartile, CI 0.09–0.57, p < 0.001), showing an improvement of the 8-year OS from 83 to 94 % (Fig. 3a). The PAM50 subtype distribution within the low quartile group (n = 181) of the proliferation score was as follows: Luminal A 76.24 %, Luminal B 6.63 %, HER2-enriched 17.13 %, and Basal-like 0 %. No benefit of paclitaxel was observed within the other PAM50 proliferation score groups when evaluated individually (data not shown), or when combined into one group (Fig. 3b), where the unadjusted HR for OS was 0.85 (CI 0.62–1.16).
Kaplan–Meier plots for overall survival comparing treatments according to the PAM50 proliferation score levels. a low proliferation score group (lowest quartile) in the GEICAM/9906 data set; b high proliferation score group (second, third, and fourth quartiles combined) in the GEICAM/9906 data set. c low proliferation score group (lowest quartile) in the CALGB/9342 and 9840 data set; d high proliferation score group (second, third, and fourth quartiles combined) in the CALGB/9342 and 9840 data set. P, paclitaxel
Relationship of the PAM50 proliferation score and paclitaxel benefit
To test the statistical validity of the relationship between the magnitude of paclitaxel benefit and the PAM50 proliferation score, a formal test of statistical interaction between proliferation score and paclitaxel treatment effect was performed. In a multivariate analysis of Cox models containing paclitaxel treatment and PAM50 proliferation score, the tests for interaction were found to be statistically significant (p = 0.006 as a continuous variable; p = 0.019 as group categories using quartile expression). In addition, a multivariate model for the interaction between PAM50 Proliferation Score and paclitaxel treatment that was adjusted for all clinical–pathological variables showed continued significance of the interaction between PAM50 proliferation score and paclitaxel treatment (Table 2).
To explore the degree of benefit from paclitaxel treatment in relationship to the PAM50 Proliferation score as a continuous function, the likelihood of OS was fit as a linear function of the PAM50 Proliferation score for both arms. Consistent with the above analysis, the magnitude of paclitaxel benefit appeared to increase continuously as the PAM50 Proliferation score decreased (Fig. 4).
Paclitaxel benefit and clinical–pathological variables
In order to identify other predictors of response to weekly paclitaxel, the interaction of paclitaxel treatment with clinical–pathological variables (age, menopausal status, histologic grade, tumor size, ER [IHC] status, PR [IHC] status, Ki-67 [IHC], and HER2 status [IHC/CISH]) was also evaluated. No significant interactions between these variables and treatment were found.
Independent evaluation of the PAM50 proliferation score and weekly paclitaxel benefit
The unexpected result in GEICAM/9906 suggested that the schedule of paclitaxel administration might be important. To independently evaluate the relationship between low Proliferation score and weekly paclitaxel benefit, we evaluated PAM50 gene expression data from 222 patients that were treated, in the metastatic setting, with either weekly paclitaxel or paclitaxel every 3 weeks (3-weekly) in the CALGB/9342 and 9840 clinical trials (online resource Fig. S2). In the combined data set, 3-weekly paclitaxel showed a decrease in the secondary endpoint of OS compared to weekly paclitaxel (unadjusted HR = 0.69, CI 0.51–0.93, p = 0.013), and the PAM50 subtypes were found to be independent predictors of OS in multivariate analysis (online resource Fig. S3A, B).
Similar to our previous observation, a benefit from weekly paclitaxel was only observed in patients whose tumors had a low PAM50 proliferation score (3-weekly vs. weekly, unadjusted HR = 2.09 within the lowest quartile, CI 1.17–3.32, p = 0.0057) (Fig. 3c, d). However, a formal test of statistical interaction between PAM50 proliferation score and treatment did not reach statistical significance (p = 0.109). Finally, the individual PAM50 subtypes were not found to be predictive of weekly paclitaxel efficacy (data not shown).
Discussion
In the era of personalized medicine, new tools that may be able to provide clinically useful prognostic and predictive information for breast cancer patients are needed [23]. Two genomic assays (OncotypeDX and Mammaprint) provide prognostic information in early breast cancer (6–8), and OncotypeDX provides predictive information of benefit from adjuvant chemotherapy (CMF or CAF) in ER-positive disease [9, 10]. However, the ability of these and other assays to predict treatment benefit to modern taxane regimens, and/or the benefit to specific drugs, remains to be determined.
A measure of proliferation is an important component of tests used for prognosis, especially in early stage ER-positive breast cancer. Proliferation is also incorporated into histologic grading, either by counting mitotic figures (i.e., modified Nottingham–Bloom–Richardson score) or by developing a mitotic index using a cell cycle regulated biomarker such as Ki-67 [24–26]. However, using more quantitative methods and many cell cycle regulated genes to assess proliferation can provide more powerful and objective prognostic information over grade or a mitotic index by Ki-67 [14]. In this study, we found that the PAM50 proliferation score signature, which is the average expression value of 11 proliferation-related genes, was predictive for benefit of weekly paclitaxel in the adjuvant setting. It is important to note that although we did not test pre-specified cutoffs of this signature in the GEICAM/9906 trial, the HR for OS in the low quartile group was highly significant in magnitude and p value (unadjusted HR = 0.232, p = 0.002). In addition, the test of interaction between paclitaxel treatment and PAM50 Proliferation score was statistically significant, even when all other clinical–pathological variables were considered. More importantly, the relationship between low proliferative status as determined by gene expression and weekly paclitaxel benefit was not excluded in an independent data set (the CALGB/9342 and 9840 clinical trials) where the interaction test did not reach statistical significance possibly due to the smaller sample size.
We and others have previously reported that the benefit of adding weekly adjuvant paclitaxel to anthracycline-based chemotherapy is small [1–4]. Thus, identification of which patients might benefit the most from this drug and schedule seems justified. Traditional clinical–pathological parameters (i.e., age, tumor size, number of positive nodes, ER status, PR status, and HER2 status) and the PAM50 intrinsic subtypes were not found to be predictive of adjuvant paclitaxel efficacy. These results are in contrast with data from the CALBG 9344/Intergroup 0148 trial where HER2 status was predictive of adjuvant paclitaxel efficacy [27]. However, the asymmetry in the duration of chemotherapy between the arms in CALGB/9344, and the difference in dose (100 vs. 175 mg/m2) and schedule (weekly vs. every 3 weeks) makes difficult the interpretation of their results in light of our present findings. In fact, other studies looking at the same relationship have reported contradictory results [28–30], and in these studies, the dose and schedule of the paclitaxel also vary; thus it is likely that when comparing paclitaxel efficacy across studies, the dose and schedule must also be taken into account.
Many other single biomarkers such as ER, tau protein, and Ki-67 have been proposed as predictors of response to taxanes or paclitaxel in particular [31–39]. Some of these studies contain significant weaknesses such as small sample sizes and lack of test standardizations, and none of them has been clinically implemented. Interestingly, in our study, the proliferation-related biomarker, Ki-67 by IHC, did not predict paclitaxel benefit despite being evaluated at a central pathology laboratory, while the 11-gene proliferation score was significant.
At a first glance, our results showing an association between low expression of proliferation-related genes and benefit from weekly paclitaxel could appear unexpected since it is generally assumed that chemotherapy is not efficacious in tumors with low proliferative activity, such as Luminal A tumors. For example, using an IHC/FISH panel to classify tumors into the various intrinsic subtypes, Hugh et al. [40] reported that the TAC (docetaxel, doxorubicin, cyclophosphamide) regimen was superior to FAC (5-fluorouracil, doxorubicin, cyclophosphamide) in Luminal B tumors, but not in Luminal A tumors. However, using the same IHC/FISH panel, we found in a prior study that the Luminal A subtype appears to derive a significant benefit from weekly paclitaxel in the GEICAM/9906 trial [16]. Although it is generally assumed that paclitaxel and docetaxel share a similar mechanism of action and, therefore, target a similar tumor population, this might not be the case as docetaxel could potentially be more efficacious in highly proliferative tumors. In fact, in a neoadjuvant randomized phase II study comparing docetaxel monotherapy to doxorubicin monotherapy, the highly-proliferative Basal tumors (as defined by the research-based PAM50 assay) were found to be especially sensitive to docetaxel [41]. Similarly, Penault-Llorca et al. [42] reported that the benefit from adjuvant docetaxel in breast cancer patients with ER-positive tumors was mainly limited to those with high proliferation as measured by Ki-67 IHC-based index. Therefore, the possibility that weekly paclitaxel versus every 3-weekly docetaxel targets different populations of tumors is a distinct hypothesis that merits further investigation.
The main target of paclitaxel seems to be the beta-tubulin protein, and its primary cellular effect is to cause abnormal stabilization of the dynamic microtubule polymerization, leading to the failure of mitosis. Other mechanisms such as generation of early reactive oxygen radicals and antiangiogenic effects have also been proposed [43–45]. It has also been suggested that the antitumor effect of paclitaxel could depend on drug exposure [46], and this could explain the increasing antitumor activity associated with weekly administrations [5]. For example, the main treatment effect we observed in GEICAM/9906 and CALGB/9342 and 9840 may be explained by Gompertzian kinetics [47]. According to this hypothesis, a near continuous dose that is given over an extended period of time is able to effectively target the slow growing tumors, but conversely this low dose never achieves a high enough level to kill the rapidly growing tumors. In order to further test this possibility and to validate the predictive value of the PAM50 proliferation score, new confirmatory studies involving patients from additional trials that evaluated weekly and non-weekly paclitaxel containing regimens like ECOG-1199 and GEICAM/2003-02 are needed.
References
De Laurentiis M, Cancello G, D’Agostino D, Giuliano M, Giordano A, Montagna E, Lauria R, Forestieri V, Esposito A, Silvestro L, Pennacchio R, Criscitiello C, Montanino A, Limite G, Bianco AR, De Placido S (2008) Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol 26:44–53
Nowak AK, Wilcken NR, Stockler MR, Hamilton A, Ghersi D (2004) Systematic review of taxane-containing versus non-taxane-containing regimens for adjuvant and neoadjuvant treatment of early breast cancer. Lancet Oncol 5:372–380
Tang SC (2009) Taxanes in the adjuvant treatment of early breast cancer, emerging consensus and unanswered questions. Cancer Invest 27:489–495
Bria E, Nistico C, Cuppone F, Carlini P, Ciccarese M, Milella M, Natoli G, Terzoli E, Cognetti F, Giannarelli D (2006) Benefit of taxanes as adjuvant chemotherapy for early breast cancer: pooled analysis of 15,500 patients. Cancer 106:2337–2344
Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, Wolff AC, Sledge GW Jr, Wood WC, Davidson NE (2008) N Engl J Med 358:1663–1671
van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R (2002) A gene expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999–2009
Buyse M, Loi S, van’t Veer L, Viale G, Delorenzi M, Glas AM, d’Assignies MS, Bergh J, Lidereau R, Ellis P, Harris A, Bogaerts J, Therasse P, Floore A, Amakrane M, Piette F, Rutgers E, Sotiriou C, Cardoso F, Piccart MJ; TRANSBIG Consortium (2006) Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst 98: 1183–1192
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxi-fen treated, node-negative breast cancer. N Engl J Med 351:2817–2826
Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE Jr, Wickerham DL, Wolmark N (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726–3734
Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF, Breast Cancer Intergroup of North America (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11:55–65
Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, Quackenbush JF, Stijleman IJ, Palazzo J, Marron JS, Nobel AB, Mardis E, Nielsen TO, Ellis MJ, Perou CM, Bernard PS (2009) Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 27:1160–1167
Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, Atkins D, Foekens JA (2005) Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 19–25;365(9460):671–679
Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B, Desmedt C, Larsimont D, Cardoso F, Peterse H, Nuyten D, Buyse M, Van de Vijver MJ, Bergh J, Piccart M, Delorenzi M (2006) Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 98(4):262–272
Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, Davies SR, Snider J, Stijleman IJ, Reed J, Cheang MC, Mardis ER, Perou CM, Bernard PS, Ellis MJ (2010) A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res 16(21):5222–5232
Martín M, Rodríguez-Lescure A, Ruiz A, Alba E, Calvo L, Ruiz-Borrego M, Munárriz B, Rodríguez CA, Crespo C, de Alava E, López García-Asenjo JA, Guitián MD, Almenar S, González-Palacios JF, Vera F, Palacios J, Ramos M, Gracia Marco JM, Lluch A, Alvarez I, Seguí MA, Mayordomo JI, Antón A, Baena JM, Plazaola A, Modolell A, Pelegrí A, Mel JR, Aranda E, Adrover E, Alvarez JV, García Puche JL, Sánchez-Rovira P, Gonzalez S, López-Vega JM, GEICAM 9906 Study Investigators (2008) Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early breast cancer. J Natl Cancer Inst 100:805–814
Martín M, Rodríguez-Lescure A, Ruiz A, Alba E, Calvo L, Ruiz-Borrego M, Santaballa A, Rodríguez CA, Crespo C, Abad M, Domínguez S, Florián J, Llorca C, Méndez M, Godes M, Cubedo R, Murias A, Batista N, García MJ, Caballero R, de Alava E (2010) Molecular predictors of efficacy of adjuvant weekly paclitaxel in early breast cancer. Breast Cancer Res Treat 123:149–157
Arup laboratories: PAM50 breast cancer intrinsic classifier information. http://www.aruplab.com/Lab-Tests/General-Oncology/PAM50/index.jsp
Elloumi F, Hu Z, Li Y, Parker JS, Gulley ML, Amos KD, Troester MA (2011) Systematic bias in genomic classification due to contaminating non-neoplastic tissue in breast tumor samples. BMC Med Genomics 4:54
Winer EP, Berry DA, Woolf S, Duggan D, Kornblith A, Harris LN, Michaelson RA, Kirshner JA, Fleming GF, Perry MC, Graham ML, Sharp SA, Keresztes R, Henderson IC, Hudis C, Muss H, Norton L (2004) Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: Cancer and Leukemia Group B trial 9342. J Clin Oncol 1 22(11):2061–2018
Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, Gipson G, Burstein H, Lake D, Shapiro CL, Ungaro P, Norton L, Winer E, Hudis C (2008) Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of cancer and leukemia group b protocol 9840. J Clin Oncol 26:1642–1649
Simon RM, Paik S, Hayes DF (2009) Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 101(21):1446–1452
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of NCI-EORTC Working Group on Cancer Diagnostics (2005) Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 23:9067–9072
Prat A, Ellis MJ, Perou CM (2011) Practical implications of gene-expression-based assays for breast oncologists. Nat Rev Clin Oncol 9(1):48–57
Simpson JF, Gray R, Dressler LG, Cobau CD, Falkson CI, Gilchrist KW, Pandya KJ, Page DL, Robert NJ (2000) Prognostic value of histologic grade and proliferative activity in axillary node-positive breast cancer: results from the Eastern Cooperative Oncology Group Companion Study, EST 4189. J Clin Oncol 18(10):2059–2069
Meyer JS, Alvarez C, Milikowski C, Olson N, Russo I, Russo J, Glass A, Zehnbauer BA, Lister K, Parwaresch R; Cooperative Breast Cancer Tissue Resource (2005) Breast carcinoma malignancy grading by Bloom–Richardson system vs proliferation index: reproducibility of grade and advantages of proliferation index. Mod Pathol. 18(8):1067–1078. Erratum in: Mod Pathol 18(12):1649
Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A’Hern R, Salter J, Detre S, Hills M, Walsh G, IMPACT Trialists Group (2007) Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 99(2):167–170
Hayes DF, Thor AD, Dressler LG (2007) HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med 357:1496–1506
Konecny GE, Thomssen C, Lück HJ, Untch M, Wang HJ, Kuhn W, Eidtmann H, du Bois A, Olbricht S, Steinfeld D, Möbus V, von Minckwitz G, Dandekar S, Ramos L, Pauletti G, Pegram MD, Jänicke F, Slamon DJ (2004) HER-2/neu gene amplification and response to paclitaxel in patients with metastatic breast cancer. J Natl Cancer Inst 96:1141–1151
Gonzalez-Angulo AM, Krishnamurthy S, Yamamura Y, Broglio KR, Pusztai L, Buzdar AU, Hortobagyi GN, Esteva FJ (2004) Lack of association between amplification of HER-2 and response to preoperative taxanes in patients with breast carcinoma. Cancer 101:258–263
Azambuja E, Durbecq V, Rosa DD, Colozza M, Larsimont D, Piccart-Gebhart M, Cardoso F (2008) HER-2 overexpression/amplification and its interaction with taxane-based therapy in breast cancer. Ann Oncol 19:223–232
Martin M, Mackey J, Vogel C (2007) Benefit from adjuvant taxanes and endocrine responsiveness in breast cancer. The Breast 16:S127–S131
Van Poznak C, Tan L, Panageas KS, Arroyo CD, Hudis C, Norton L, Seidman AD (2002) Assessment of molecular markers of clinical sensitivity to single-agent taxane therapy for metastatic breast cancer. J Clin Oncol 20:2319–2326
Harris LN, Broadwater G, Lin NU, Miron A, Schnitt SJ, Cowan D, Lara J, Bleiweiss I, Berry D, Ellis M, Hayes DF, Winer EP, Dressler L (2006) Molecular subtypes of breast cancer in relation to paclitaxel response, outcomes in women with metastatic disease: results from CALGB 9342. Breast Cancer Res 8(6):R66
Rouzier R, Rajan R, Wagner P, Hess KR, Gold DL, Stec J, Ayers M, Ross JS, Zhang P, Buchholz TA, Kuerer H, Green M, Arun B, Hortobagyi GN, Symmans WF, Pusztai L (2005) Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. PNAS 102:8315–8320
Galmarini CM, Treilleux I, Cardoso F, Bernard-Marty C, Durbecq V, Gancberg D, Bissery MC, Paesmans M, Larsimont D, Piccart MJ, Di Leo A, Dumontet C (2008) Class III btubulin isotype predicts response in adjuvant breast cancer patients randomly treated either with single-agent doxorubicin or docetaxel. Clin Cancer Res 14:4511–4516
Hasegawa S, Miyoshi Y, Egawa C, Ishitobi M, Taguchi T, Tamaki Y, Monden M, Noguchi S (2003) Prediction of response to docetaxel by quantitative analysis of class I and III beta tubulin isotype mRNA expression in human breast cancers. Clin Cancer Res 9:2992–2997
Bernard-Marty C, Treilleux I, Dumontet C, Cardoso F, Fellous A, Gancberg D, Bissery MC, Paesmans M, Larsimont D, Piccart MJ, Di Leo A (2002) Microtubule- associated parameters as predictive markers of docetaxel activity in advanced breast cancer patients: results of a pilot study. Clin Cancer Breast 3:341–345
Pusztai L, Jeong JH, Gong Y, Ross JS, Kim C, Paik S, Rouzier R, Andre F, Hortobagyi GN, Wolmark N, Symmans WF (2009) Evaluation of microtubule-associated protein-tau expression as a prognostic and predictive marker in the NSABP-B 28 randomized clinical trial. J Clin Oncol 27:4287–4292
Yang SX, Costantino JP, Kim C, Mamounas EP, Nguyen D, Jeong JH, Wolmark N, Kidwell K, Paik S, Swain SM (2010) Akt phosphorylation at Ser473 predicts benefit of paclitaxel chemotherapy in node-positive breast cancer. J Clin Oncol 28:2974–2981
Hugh J, Hanson J, Cheang MC, Nielsen TO, Perou CM, Dumontet C, Reed J, Krajewska M, Treilleux I, Rupin M, Magherini E, Mackey J, Martin M, Vogel C (2009) Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol 27:1168–1176
Martin M, Romero A, Cheang MC, López García-Asenjo JA, García-Saenz JA, Oliva B, Román JM, He X, Casado A, de la Torre J, Furio V, Puente J, Caldés T, Vidart JA, Lopez-Tarruella S, Diaz-Rubio E, Perou CM (2011) Genomic predictors of response to doxorubicin versus docetaxel in primary breast cancer. Breast Cancer Res Treat 128:127–136
Penault-Llorca F, André F, Sagan C, Lacroix-Triki M, Denoux Y, Verriele V, Jacquemier J, Baranzelli MC, Bibeau F, Antoine M, Lagarde N, Martin AL, Asselain B, Roché H (2009) Ki67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer. J Clin Oncol 27:2809–2815
Horwitz SB (1994) Taxol (paclitaxel): mechanisms of action. Ann Oncol 5(Suppl 6):S3–S6
Lau DH, Xue L, Young LJ, Burke PA, Cheung AT (1999) Paclitaxel (Taxol): an inhibitor of angiogenesis in a highly vascularized transgenic breast cancer. Cancer Biother Radiopharm 14:31–36
Alexandre J, Hu Y, Lu W, Pelicano H, Huang P (2007) Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res 67:3512–3517
Yeung TK, Germond C, Chen X, Wang Z (1999) The mode of action of taxol: apoptosis at low concentration and necrosis at high concentration. Biochem Biophys Res Commun 263:398–404
Norton L (1988) A gompertzian model of human breast cancer growth. Cancer Res 48:7067–7071
Acknowledgments
The authors would like to thank Torsten O. Nielsen of the Genetic Pathology Evaluation Centre, Vancouver Coastal Health Research Institute, British Columbia Cancer Agency, and University of British Columbia, Vancouver, Canada, for his critical review of the manuscript. Funding for M Martin was also supported by FEDER (RETICC-RD12/0036/0076). Funding for MJ Ellis, CM Perou, and PS Bernard was supported by the National Cancer Institute (NCI) Strategic Partnering to Evaluate Cancer Signatures Grant CA114722-01, and CM Perou was also supported by the NCI Breast SPORE program (P50-CA58223-09A1) and the Breast Cancer Research Foundation. Funding for L. Harris was supported by the National Institute of Health (NIH) Research Project Grant Program (R01). A Prat is supported by a grant from the Sociedad Española de Oncología Médica (SEOM), and is affiliated to the Medicine PhD program of the Autonomous University of Barcelona (UAB), Spain.
Disclosures
The following authors declared: MJE, CMP, and PSB consultant/advisory role at University Genomics Inc and Bioclassifier LLC and stock ownership at University Genomics Inc and Bioclassifier LLC. Rest of the authors declared no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Martín, M., Prat, A., Rodríguez-Lescure, Á. et al. PAM50 proliferation score as a predictor of weekly paclitaxel benefit in breast cancer. Breast Cancer Res Treat 138, 457–466 (2013). https://doi.org/10.1007/s10549-013-2416-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2416-2