Abstract
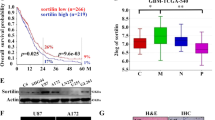
Glioblastoma (GBM) is the most common and most aggressive central nervous system tumor in adults. Due to GBM cell invasiveness and resistance to chemotherapy, current medical interventions are not satisfactory, and the prognosis for GBM is poor. It is necessary to investigate the underlying mechanism of GBM metastasis and drug resistance so that more effective treatments can be developed for GBM patients. sushi repeat-containing protein, X-linked 2 (SRPX2) is a prognostic biomarker in many different cancer cell lines and is associated with poor prognosis in cancer patients. SRPX2 overexpression promotes interactions between tumor and endothelial cells, leading to tumor progression and metastasis. We hypothesize that SRPX2 also contributes to GBM chemotherapy resistance and metastasis. Our results revealed that GBM tumor samples from 42 patients expressed higher levels of SRPX2 than the control normal brain tissue samples. High-SRPX2 expression levels are correlated with poor prognosis in those patients, as well as resistance to temozolomide in cultured GBM cells. Up-regulating SRPX2 expression in cultured GBM cell lines facilitated invasiveness and migration of GBM cells, while down-regulating SRPX2 through RNA interference was inhibitory. These results suggest that SRPX2 plays an important role in GBM metastasis. Epithelial to mesenchymal transition (EMT) is one of the processes that facilitate GBM metastasis and resistance to chemotherapy. EMT marker expression was decreased in SRPX2 down-regulated GBM cells, and MAPK signaling pathway marker expression was also decreased when SRPX2 is knocked down in GBM-cultured cells. Blocking the MAPK signaling pathway inhibited GBM metastasis but did not inhibit cell invasion and migration in SRPX2 down-regulated cells. Our results indicate that SRPX2 facilitates GBM metastasis by enhancing the EMT process via the MAPK signaling pathway.







Similar content being viewed by others
References
Boulay JL, Dennefeld C, Alberga A (1987) The Drosophila developmental gene snail encodes a protein with nucleic acid binding fingers. Nature 330(6146):395–398
Frisch SM, Schaller M, Cieply B (2013) Mechanisms that link the oncogenic epithelial-mesenchymal transition to suppression of anoikis. J Cell Sci 126:21–29
Gui T, Su YJ, Shiokado A, Muragaki Y (2012) The roles of mitogen-activated protein kinase pathways in TGF-β-induced epithelial-mesenchymal transition. J Signal Transduct, Epub
Han SP, Kim JH, Han ME, Sim HE, Kim KS, Yoon S, Baek SY, Kim BS, Oh SO (2011) SNAIL is involved in the proliferation and migration of glioblastoma cells. Cell Mol Neurobiol 31(3):489–496
Huang RY, Chung VY, Thiery JP (2012) Targeting pathways contributing to epithelial-mesenchymal transition (EMT) in epithelial ovarian cancer. Curr Drug Targ 13:1649–1653
Jiao Y, Li H, Liu Y, Guo A, Xu X, Qu X, Wang S, Zhao J, Li Y, Cao Y (2015) Resveratrol inhibits the invasion of glioblastoma-initiating cells via down-regulation of the PI3K/Akt/NF-κB signaling pathway. Nutrients 7(6):4383–4402
Jordan NV, Johnson GL, Abell AN (2011) Tracking the intermediate stages of epithelial-mesenchymal transition in epithelial stem cells and cancer. Cell Cycle 10:2865–2873
Kahlert UD, Nikkhah G, Maciaczyk J (2013) Epithelial-to-mesenchymal(-like) transition as a relevant molecular event in malignant gliomas. Cancer Lett 331(2):131–138
Kurosawa H, Goi K, Inukai T, Inaba T, Chang KS, Shinjyo T, Rakestraw KM, Naeve CW (1999) Two candidate downstream target genes for E2A-HLF. Blood 93:321–332
Laure L, Bellacosa A (2005) Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3’ kinase/AKT pathways. Oncogene 24:7443–7454
Lee K, Nelson CM (2012) New insights into the regulation of epithelial-mesenchymal transition and tissue fibrosis. Int Rev Cell Mol Biol 294:171–221
Lee JM, Dedhar S, Kalluri R, Thompson EW (2006) The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 72:973–981
Lee Y, Lee M, Kim S (2013) Gas6 induces cancer cell migration and epithelial-mesenchymal transition through upregulation of MAPK and Slug. Biochem Biophys Res Comm 434(1):8–14
Liu KL, Wu J, Zhou Y, Fan JH (2015) Increased Sushi repeat-containing protein X-linked 2 is associated with progression of colorectal cancer. Med Oncol 32(4):99
Myung JK, Choi SA, Kim SK, Wang KC, Park SH (2014) Snail plays an oncogenic role in glioblastoma by promoting epithelial mesenchymal transition. Int J Clin Exp Pathol 7(5):1977–1987
Nieto MA, Sargent MG, Wilkinson DG, Cooke J (1994) Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264(5160):835–839
O’Leary JM, Bromek K, Black GM, Uhrinova S, Schmitz C, Wang X, Krych M, Atkinson JP, Uhrin D, Barlow PN (2004) Backbone dynamics of complement control protein (CCP) modules reveals mobility in binding surfaces. Protein Sci 13:1238–1250
Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA (2008) Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 68(10):3645–3654
Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7(6):415–428
Salmi M, Bruneau N, Cillario J, Lozovaya N, Massacrier A, Buhler E, Cloarec R, Tsintsadze T, Watrin F, Tsintsadze V, Zimmer C, Villard C, Lafitte D, Cardoso C, Bao L, Lesca G, Rudolf G, Muscatelli F, Pauly V, Khalilov I, Durbec P, Ben-Ari Y, Burnashev N, Represa A, Szepetowski P (2013) Tubacin prevents neuronal migration defects and epileptic activity caused by rat Srpx2 silencing in utero. Brain 136(Pt 8):2457–2473
Soares DC, Gerloff DL, Syme NR, Coulson AF, Parkinson J, Barlow PN (2005) Large-scale modelling as a route to multiple surface comparisons of the CCP module family. Protein Eng Des Sel 18:379–388
Tam WL, Weinberg RA (2013) The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med 19:1438–1449
Tanaka K, Arao T, Maegawa M, Matsumoto K, Kaneda H, Kudo K, Fujita Y, Yokote H, Yanagihara K, Yamada Y, Okamoto I, Nakagawa K, Nishio K (2009) SRPX2 is overexpressed in gastric cancer and promotes cellular migration and adhesion. Int J Cancer 124(5):1072–1080
Tanaka K, Arao T, Tamura D, Aomatsu K, Furuta K, Matsumoto K, Kaneda H, Kudo K, Fujita Y, Kimura H, Yanagihara K, Yamada Y, Okamoto I, Nakagawa K, Nishio K (2012) SRPX2 is a novel chondroitin sulfate proteoglycan that is overexpressed in gastrointestinal cancer. PLoS One 7(1):e27922
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890
Tso CL, Shintaku P, Chen J, Liu LJ, Chen Z, Yoshimoto K, Mischel PS, Cloughesy TF, Liau LM, Nelson SF (2006) Primary glioblastomas express mesenchymal stem-like properties. Mol Cancer Res 4(9):607–619
Voulgari A, Pintzas A (2009) Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta 1796(2):75–90
Author information
Authors and Affiliations
Corresponding author
Additional information
Haitao Tang and Jiaxin Zhao have contributed equally to the work.
Rights and permissions
About this article
Cite this article
Tang, H., Zhao, J., Zhang, L. et al. SRPX2 Enhances the Epithelial–Mesenchymal Transition and Temozolomide Resistance in Glioblastoma Cells. Cell Mol Neurobiol 36, 1067–1076 (2016). https://doi.org/10.1007/s10571-015-0300-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-015-0300-9