Abstract
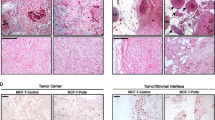
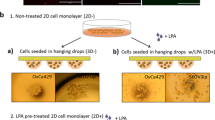
High grade serous ovarian tumors often metastasize transperitoneally, a process that begins when small tumor nodules de-adhere and are released into the fluid of the abdominal cavity where they float freely to reach new sites on the peritoneal wall. Podocalyxin, a small anti-adhesive sialomucin, has been shown to contribute to non-adhesive membrane domain formation in some epithelia and is overexpressed in a variety of cancers. We therefore assessed podocalyxin expression on a previously characterized tissue microarray and found that 87% (169/194) of high grade serous epithelial ovarian carcinomas were positive for podocalyxin. In addition, cell surface localization of podocalyxin was associated with a significant decrease in disease-free survival in these tumors. When podocalyxin was force-expressed in serous ovarian carcinoma-derived OVCAR-3 cells it was targeted to the cell surface and it decreased the adhesion of these cells to mesothelial monolayers, fibronectin and immobilized β1 integrin-binding antibodies. This decrease in adhesion was associated with a modest decrease in cell surface β1 integrin. In monolayer culture, podocalyxin was targeted to the free, apical domains of OVCAR-3 cells and it appeared to decrease β1 integrin levels on the attached basolateral domains of the same cells. Furthermore, in 3-dimensional basement membrane gel culture, the cells formed small, cohesive nodules and podocalyxin localized to membrane domains at the cell–basement membrane interface. Therefore, podocalyxin’s ability to facilitate the formation of non-adhesive membrane domains may contribute to the formation of free-floating high grade serous tumor nodules during the initial steps of transperitoneal metastasis.








Similar content being viewed by others
References
Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ, American Cancer Society (2004) Cancer statistics. CA Cancer J Clin 54:8–29
Trimble EL, Davis J, Disaia P, Fujiwara K, Gaffney D, Kristensen G, Ledermann J, Pfisterer J, Quinn M, Reed N, Schoenfeldt M, Thigpen JT, Gynecologic Cancer Intergroup (2007) Clinical trials in gynecological cancer. Int J Gynecol Cancer 17(3):547–556
Flam F, Einhorn N, Sjovall K (1988) Symptomatology of ovarian cancer. Eur J Obstet Gynecol Reprod Biol 27:53–57
Davidson B, Risberg B, Reich R, Berner A (2003) Effusion cytology in ovarian cancer: new molecular methods as aids to diagnosis and prognosis. Clin Lab Med 23:729–754
Schopperle WM, Kershaw DB, DeWolf WC (2003) Human embryonal carcinoma tumor antigen, Gp200/GCTM-2, is podocalyxin. Biochem Biophys Res Commun 300(2):285–290
Kelley TW, Huntsman D, McNagny KM, Roskelley CD, Hsi ED (2005) Podocalyxin: a marker of blasts in acute leukemia. Am J Clin Pathol 124(1):134–142
Ney JT, Zhou H, Sipos B, Buttner R, Chen X, Kloppel G, Gutgemann I (2007) Podocalyxin-like protein 1 expression is useful to differentiate pancreatic ductal adenocarcinomas from adenocarcinomas of the biliary and gastrointestinal tracts. Hum Pathol 38(2):359–364
Schopperle WM, DeWolf WC (2007) The TRA-1-60 and TRA-1-81 human pluripotent stem cell markers are expressed on podocalyxin in embryonal carcinoma. Stem Cells 25(3):723–730
Yasuoka H, Tsujimoto M, Hirokawa M, Tori M, Nakahara M, Miyauchi A, Kodama R, Sanke T, Nakamura Y (2008) Podocalyxin expression in undifferentiated thyroid carcinomas. J Clin Pathol 61(11):1228–1229
Thomas SN, Schnaar RL, Konstantopoulos K (2009) Podocalyxin-like protein is an E-/L-selectin ligand on colon carcinoma cells: comparative biochemical properties of selectin ligands in host and tumor cells. Am J Physiol Cell Physiol 296(3):C505–C513
Hsu YH, Lin WL, Hou YT, Pu YS, Shun CT, Chen CL, Wu YY, Chen JY, Chen TH, Jou TS (2010) Podocalyxin EBP50 ezrin molecular complex enhances the metastatic potential of renal cell carcinoma through recruiting Rac1 guanine nucleotide exchange factor ARHGEF7. Am J Pathol 176(6):3050–3061
Casey G, Neville PJ, Liu X, Plummer SJ, Cicek MS, Krumroy LM, Curran AP, McGreevy MR, Catalona WJ, Klein EA, Witte JS (2006) Podocalyxin variants and risk of prostate cancer and tumor aggressiveness. Hum Mol Genet 15(5):735–741
Somasiri A, Nielsen JS, Makretsov N, McCoy ML, Prentice L, Gilks CB, Chia SK, Gelmon KA, Kershaw DB, Huntsman DG, McNagny KM, Roskelley CD (2004) Overexpression of the anti-adhesin podocalyxin is an independent predictor of breast cancer progression. Cancer Res 64(15):5068–5073
Cheung H-H, Davis AJ, Lee T-L, Pang AL, Nagrani S, Rennert OM, Chan W-Y (2011) Methylation of an intronic region regulates miR-199a in testicular tumor malignancy. Oncogene 30(31):3404–3415
Kerjaschki D, Sharkey DJ, Farquhar MG (1984) Identification and characterization of podocalyxin-the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol 98:1591–1596
Dekan G, Miettinen A, Schnabel E, Farquhar MG (1990) Binding of monoclonal antibodies to glomerular endothelium, slit membranes, and epithelium after in vivo injection. Localization of antigens and bound IgGs by immunoelectron microscopy. Am J Pathol 137(4):913–927
Doyonnas R, Kershaw DB, Duhme C, Merkens H, Chelliah S, Graf T, McNagny KM (2001) Anuria, omphalocele, and perinatal lethality in mice lacking the CD-34-related protein podocalyxin. J Exp Med 194:13–27
Miettinen A, Solin ML, Reivinen J, Juvonen E, Vaisanen R, Holthofer H (1999) Podocalyxin in rat platelets and megakaryocytes. Am J Pathol 154(3):813–822
Vitureira N, McNagny K, Soriano E, Burgaya F (2005) Pattern of expression of the podocalyxin gene in the mouse brain during development. Gene Expr Patterns 5(3):349–354
Meder D, Shevchenko A, Simons K, Füllekrug J (2005) Gp135/podocalyxin and NHERF-2 participate in the formation of a preapical domain during polarization of MDCK cells. J Cell Biol 168(2):303–313
Nielsen JS, McCoy M, Chelliah S, Vogl AW, Roskelley CD, McNagny KM (2007) The CD34-related molecule podocalyxin is a potent inducer of microvillus formation. PLoS ONE 2:e237
Strilic B, Kucera T, Eglinger J, Hughes MR, McNagny KM, Tsukita S, Dejana E, Ferrara Lammert E (2009) The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell 17(4):505–515
Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC (2001) Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev 22(2):255–288
Kurman RJ, Shih IeM (2010) The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 34(3):433–443
Jarboe E, Folkins A, Nucci MR, Kindelberger D, Drapkin R, Miron A, Lee Y, Crum CP (2008) Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol 27(1):1–9
Kershaw DB, Beck SG, Wharram BL, Wiggins JE, Goyal M, Thomas PE, Wiggins RC (1997) Molecular cloning and characterization of human podocalyxin-like protein. J Biol Chem 272(25):15708–15714
Gilks CB, Ionescu DN, Kalloger SE, Köbel M, Irving J, Clarke B, Santos J, Le N, Moravan V, Swenerton K, Cheryl Brown Ovarian Cancer Outcomes Unit of the British Columbia Cancer Agency (2008) Tumor cell type can be reproducibly diagnosed and is of independent prognostic significance in patients with maximally debulked ovarian carcinoma. Hum Pathol 39(8):1239–1251
Köbel M, Kalloger SE, Boyd N, McKinney S, Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, Prentice LM, Miller D, Santos J, Swenerton K, Gilks CB, Huntsman D (2008) Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med 5(12):e232
Graves ML, Zhou L, MacDonald G, Mueller CR, Roskelley CD (2007) Regulation of the BRCA1 promoter in ovarian surface epithelial cells and ovarian carcinoma cells. FEBS Lett 581(9):1825–1833
Connell ND, Rheinwald JG (1983) Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell 34(1):245–253
Pinkas J, Leder P (2002) Mek1 signaling mediates transformation and metastasis of EpH4 mammary epithelial cells independent of epithelial to mesenchymal transition. Cancer Res 62:4781–4790
Hamilton TC, Young RC, McKoy WM, Grotzinger KR, Green JA, Chu EW, Whang-Peng J, Rogan AM, Green WR, Ozols RF (1983) Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res 43(11):5379–5389
Cannistra SA, Ottensmeier C, Niloff J, Orta B, DiCarlo J (1995) Expression and function of β1 and αvβ3 integrins in ovarian cancer. Gynecol Oncol 58:216–225
Rangarajan S, Enserink JM, Kuiperij HB, de Rooij J, Price LS, Schwede F, Bos JL (2003) Cyclic AMP induces integrin-mediated adhesion through Epac and Rap1 upon stimulation of the β2-adrenergic receptor. J Cell Biol 160:487–493
Takeda T, Go WY, Orlando RA, Farquhar MG (2000) Expression of podocalyxin inhibits cell–cell adhesion and modifies junctional properties in madin-Darby Canine Kidney Cells. Mol Biol Cell 11:3219–3232
Abdullah KM, Udoh EA, Shewen PE, Mellors A (1992) A neutral glycoprotease of Pasteurella haemolytica A1 specifically cleaves O-sialoglycoproteins. Infect Immun 60:56–62
Burleson KM, Casey RC, Skubitz KM, Pambuccian SE, Oegema TR Jr, Skubitz AP (2004) Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol 93(1):170–181
Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, Lee Y (2007) The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol 19(1):3–9
Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, Feltmate CM, Berkowitz RS, Muto MG (2007) Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol 25(25):3985–3990
Fountain J, Trimble E, Birrer MJ (2006) Summary and discussion of session recommendations. Gynecol Oncol 103(2, Suppl 1):S23–S25
Francis SS, Sfakianos J, Lo B, Mellman I (2011) A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. J Cell Biol 193:219–233
Li Y, Li J, Straight SW, Kershaw DB (2002) PDZ domain-mediated interaction of rabbit podocalyxin and Na(+)/H(+) exchange regulatory factor-2. Am J Physiol Renal Physiol 282:F1129–F1139
Tan PC, Furness SG, Merkens H, Lin S, McCoy ML, Roskelley CD, Kast J, McNagny KM (2006) Na+/H+ exchanger regulatory factor-1 is a hematopoietic ligand for a subset of the CD34 family of stem cell surface proteins. Stem Cells 24:1150–1161
Orlando RA, Takeda T, Zak B, Schmieder S, Benoit VM, McQuistan T, Furthmayr H, Farquhar MG (2001) The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol 12:1589–1598
Takeda T, McQuistran T, Orlando RA, Farquhar MG (2001) Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest 108:289–301
Sizemore S, Cicek M, Sizemore N, Ng KP, Casey G (2007) Podocalyxin increases the aggressive phenotype of breast and prostate cancer cells in vitro through its interaction with ezrin. Cancer Res 67(13):6183–6191
Meng X, Ezzati P, Wilkins JA (2011) Requirement of podocalyxin in TGF-beta induced epithelial mesenchymal transition. PLoS ONE 6(4):e18715
Kobel M, Gradhand E, Zeng K, Schmitt WD, Kriese K, Lantzsch T, Wolters M, Dittmer J, Strauss HG, Thomssen C, Hauptmann S (2006) Ezrin promotes ovarian carcinoma cell invasion and its retained expression predicts poor prognosis in ovarian carcinoma. Int J Gynecol Pathol 25(2):121–130
Tabrizi AD, Kalloger SE, Köbel M, Cipollone J, Roskelley CD, Mehl E, Gilks CB (2010) Primary ovarian mucinous carcinoma of intestinal type: significance of pattern of invasion and immunohistochemical expression profile in a series of 31 cases. Int J Gynecol Pathol 29(2):99–107
Klausen C, Leung PC, Auersperg N (2009) Cell motility and spreading are suppressed by HOXA4 in ovarian cancer cells: possible involvement of β1 integrin. Mol Cancer Res 7(9):1425–1437
Acknowledgments
M.G. was the recipient University of British Columbia and Babicki family graduate scholarships. M.K. was an Eli Lilly Canada Oncology Fellow and KMM is a Michael Smith Foundation for Health Research Scholar. This work was funded by a grant from the Canadian Institutes of Health Research to C.D.R and K.M.M.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cipollone, J.A., Graves, M.L., Köbel, M. et al. The anti-adhesive mucin podocalyxin may help initiate the transperitoneal metastasis of high grade serous ovarian carcinoma. Clin Exp Metastasis 29, 239–252 (2012). https://doi.org/10.1007/s10585-011-9446-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-011-9446-0