Abstract
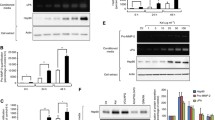
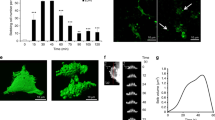
Elastin-rich lung extracellular matrix is largely remodeled during tumor invasion. Elastin degradation produces peptides displaying a wide range of biological activities. These elastin derived peptides (EP) interact with the elastin receptor complex (ERC) but also bind to αVβ3 integrin and galectin-3. In this study, we explored the role of EP and their receptors in tumor progression of lung carcinomas. Non-invasive and invasive lung tumor cell lines were incubated in presence of kappa-elastin (κE) or with synthetic peptides displaying receptor-specific sequences (VGVAPG, GRKRK, AGVPGLGVG and AGVPGFGAG). Modified Boyden chamber assays revealed an increased invasive capacity of invasive cells induced by κE. EP treatment had no effect on cell proliferation but zymography analysis revealed an increase of pro-MMP-2 and uPA levels in the conditioned media of treated cells. Moreover, the active form of MMP-2 was increased in invasive cells. Interestingly, this regulation was not observed at the mRNA level and actinomycin D was unable to inhibit κE effects. We also observed that the regulation of proteases protein level following κE treatment was an early process detectable after 1 h. All these effects could not be inhibited by lactose and V14, two ERC antagonists, or by blocking antibodies against αVβ3 integrin and galectin-3. Finally, VGVAPG and GRKRK failed to reproduce κE effects whereas nonapeptides partially mimicked them. These results demonstrate that treatment with EP up-regulates invasiveness of lung tumor cells via the release of proteolytic enzymes. This modulation involves post-transcriptional mechanisms and a nonapeptide-receptor different from the ERC, αVβ3 integrin and galectin-3.






Similar content being viewed by others
Abbreviations
- ACTD:
-
Actinomycin D
- AGVPGFGAG:
-
Ala-Gly-Val-Pro-Gly-Phe-Gly-Ala-Gly
- AGVPGLGVG:
-
Ala-Gly-Val-Pro-Gly-Leu-Gly-Val-Gly
- CD:
-
Circular dichroism
- EBP:
-
Elastin binding protein
- ECM:
-
Extracellular matrix
- EDTA:
-
Ethylenediaminetetraacetic acid
- EP:
-
Elastin derived peptides
- ERC:
-
Elastin receptor complex
- GRKRK:
-
Gly-Arg-Lys-Arg-Lys
- κE:
-
Kappa-elastin
- MMP:
-
Matrix metalloproteinase
- Neu-1:
-
Neuraminidase-1
- NMR:
-
Nuclear magnetic resonance
- PMSF:
-
Phenylmethylsulfonyl fluoride
- PPCA:
-
Protective protein/cathepsin A
- S-Gal:
-
Spliced galactosidase
- uPA:
-
Urokinase plasminogen activator
- VGVAPG:
-
Val-Gly-Val-Ala-Pro-Gly
References
Kielty CM, Sherratt MJ, Shuttleworth CA (2002) Elastic fibres. J Cell Sci 115:2817–2828
Mithieux SM, Weiss AS (2005) Elastin. Adv Protein Chem 70:437–461
Shapiro SD, Endicott SK, Province MA et al (1991) Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of d-aspartate and nuclear weapons-related radiocarbon. J Clin Invest 87:1828–1834
Werb Z, Banda MJ, McKerrow JH et al (1982) Elastases and elastin degradation. J Invest Dermatol 79(Suppl 1):154s–159s
Lapis K, Tímár J (2002) Role of elastin–matrix interactions in tumor progression. Semin Cancer Biol 12:209–217
Mecham RP, Hinek A, Griffin GL et al (1989) The elastin receptor shows structural and functional similarities to the 67-kDa tumor cell laminin receptor. J Biol Chem 264:16652–16657
Mochizuki S, Brassart B, Hinek A (2002) Signaling pathways transduced through the elastin receptor facilitate proliferation of arterial smooth muscle cells. J Biol Chem 277:44854–44863
Robinet A, Fahem A, Cauchard J-H et al (2005) Elastin-derived peptides enhance angiogenesis by promoting endothelial cell migration and tubulogenesis through upregulation of MT1-MMP. J Cell Sci 118:343–356
Maeda I, Mizoiri N, Briones MPP et al (2007) Induction of macrophage migration through lactose-insensitive receptor by elastin-derived nonapeptides and their analog. J Pept Sci 13:263–268
Grosso LE, Scott M (1993) PGAIPG, a repeated hexapeptide of bovine and human tropoelastin, is chemotactic for neutrophils and Lewis lung carcinoma cells. Arch Biochem Biophys 305:401–404
Hauck M, Seres I, Kiss I et al (1995) Effects of synthesized elastin peptides on human leukocytes. Biochem Mol Biol Int 37:45–55
Senior RM, Griffin GL, Mecham RP et al (1984) Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J Cell Biol 99:870–874
Debret R, Antonicelli F, Theill A et al (2005) Elastin-derived peptides induce a T-helper type 1 polarization of human blood lymphocytes. Arterioscler Thromb Vasc Biol 25:1353–1358
Brassart B, Randoux A, Hornebeck W et al (1998) Regulation of matrix metalloproteinase-2 (gelatinase A, MMP-2), membrane-type matrix metalloproteinase-1 (MT1-MMP) and tissue inhibitor of metalloproteinases-2 (TIMP-2) expression by elastin-derived peptides in human HT-1080 fibrosarcoma cell line. Clin Exp Metastasis 16:489–500
Ntayi C, Labrousse A-L, Debret R et al (2004) Elastin-derived peptides upregulate matrix metalloproteinase-2-mediated melanoma cell invasion through elastin-binding protein. J Invest Dermatol 122:256–265
Jung S, Hinek A, Tsugu A et al (1999) Astrocytoma cell interaction with elastin substrates: implications for astrocytoma invasive potential. Glia 25:179–189
Hinek A, Jung S, Rutka JT (1999) Cell surface aggregation of elastin receptor molecules caused by suramin amplified signals leading to proliferation of human glioma cells. Acta Neuropathol 97:399–407
Pocza P, Süli-Vargha H, Darvas Z et al (2008) Locally generated VGVAPG and VAPG elastin-derived peptides amplify melanoma invasion via the galectin-3 receptor. Int J Cancer 122:1972–1980
Long MM, King VJ, Prasad KU et al (1989) Elastin repeat peptides as chemoattractants for bovine aortic endothelial cells. J Cell Physiol 140:512–518
Brassart B, Fuchs P, Huet E et al (2001) Conformational dependence of collagenase (matrix metalloproteinase-1) up-regulation by elastin peptides in cultured fibroblasts. J Biol Chem 276:5222–5227
Maquart F-X, Pasco S, Ramont L et al (2004) An introduction to matrikines: extracellular matrix-derived peptides which regulate cell activity. Implication in tumor invasion. Crit Rev Oncol Hematol 49:199–202
Bisaccia F, Morelli MA, De Biasi M et al (1994) Migration of monocytes in the presence of elastolytic fragments of elastin and in synthetic derivates. Structure–activity relationships. Int J Pept Protein Res 44:332–341
Castiglione Morelli MA, Bisaccia F, Spisani S et al (1997) Structure–activity relationships for some elastin-derived peptide chemoattractants. J Pept Res 49:492–499
Bisaccia F, Castiglione-Morelli MA, Spisani S et al (1998) The amino acid sequence coded by the rarely expressed exon 26A of human elastin contains a stable beta-turn with chemotactic activity for monocytes. Biochemistry 37:11128–11135
Hinek A, Wrenn DS, Mecham RP et al (1988) The elastin receptor: a galactoside-binding protein. Science 239:1539–1541
Mecham RP, Hinek A, Entwistle R et al (1989) Elastin binds to a multifunctional 67-kilodalton peripheral membrane protein. Biochemistry 28:3716–3722
Privitera S, Prody CA, Callahan JW et al (1998) The 67-kDa enzymatically inactive alternatively spliced variant of beta-galactosidase is identical to the elastin/laminin-binding protein. J Biol Chem 273:6319–6326
Duca L, Blanchevoye C, Cantarelli B et al (2007) The elastin receptor complex transduces signals through the catalytic activity of its Neu-1 subunit. J Biol Chem 282:12484–12491
Floquet N, Héry-Huynh S, Dauchez M et al (2004) Structural characterization of VGVAPG, an elastin-derived peptide. Biopolymers 76:266–280
Fuchs PFJ, Bonvin AMJJ, Bochicchio B et al (2006) Kinetics and thermodynamics of type VIII beta-turn formation: a CD, NMR, and microsecond explicit molecular dynamics study of the GDNP tetrapeptide. Biophys J 90:2745–2759
Moroy G, Alix AJP, Héry-Huynh S (2005) Structural characterization of human elastin derived peptides containing the GXXP sequence. Biopolymers 78:206–220
Plow EF, Haas TA, Zhang L et al (2000) Ligand binding to integrins. J Biol Chem 275:21785–21788
Humphries JD, Byron A, Humphries MJ (2006) Integrin ligands at a glance. J Cell Sci 119:3901–3903
Bax DV, Rodgers UR, Bilek MMM et al (2009) Cell adhesion to tropoelastin is mediated via the C-terminal GRKRK motif and integrin alphaVbeta3. J Biol Chem 284:28616–28623
Ochieng J, Furtak V, Lukyanov P (2004) Extracellular functions of galectin-3. Glycoconj J 19:527–535
Wang Y, Nangia-Makker P, Tait L et al (2009) Regulation of prostate cancer progression by galectin-3. Am J Pathol 174:1515–1523
Ochieng J, Warfield P, Green-Jarvis B et al (1999) Galectin-3 regulates the adhesive interaction between breast carcinoma cells and elastin. J Cell Biochem 75:505–514
Devy J, Duca L, Cantarelli B et al (2010) Elastin-derived peptides enhance melanoma growth in vivo by upregulating the activation of Mcol-A (MMP-1) collagenase. Br J Cancer 103:1562–1570
Jacob M-P, Hornebeck W (1985) Isolation and characterization of insoluble and kappa-elastins. Front Matrix Biol 10:92–129
Irizarry RA, Hobbs B, Collin F et al (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264
Hinek A (1996) Biological roles of the non-integrin elastin/laminin receptor. Biol Chem 377:471–480
Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11:85–95
Timar J, Lapis K, Fulop T et al (1991) Interaction between elastin and tumor cell lines with different metastatic potential; in vitro and in vivo studies. J Cancer Res Clin Oncol 117:232–238
Timár J, Diczházi C, Ladányi A et al (1995) Interaction of tumour cells with elastin and the metastatic phenotype. Ciba Found Symp 192:321–335; discussion 335–337
Martinella-Catusse C, Polette M, Noel A et al (2001) Down-regulation of MT1-MMP expression by the alpha3 chain of type IV collagen inhibits bronchial tumor cell line invasion. Lab Invest 81:167–175
Niki T, Kohno T, Iba S et al (2002) Frequent co-localization of Cox-2 and laminin-5 gamma2 chain at the invasive front of early-stage lung adenocarcinomas. Am J Pathol 160:1129–1141
Chang C, Werb Z (2001) The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol 11:S37–S43
Polette M, Nawrocki-Raby B, Gilles C et al (2004) Tumour invasion and matrix metalloproteinases. Crit Rev Oncol Hematol 49:179–186
Sidenius N, Blasi F (2003) The urokinase plasminogen activator system in cancer: recent advances and implication for prognosis and therapy. Cancer Metastasis Rev 22:205–222
Senior RM, Griffin GL, Fliszar CJ et al (1991) Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem 266:7870–7875
Dashevsky O, Varon D, Brill A (2009) Platelet-derived microparticles promote invasiveness of prostate cancer cells via upregulation of MMP-2 production. Int J Cancer 124:1773–1777
Angelucci A, D’Ascenzo S, Festuccia C et al (2000) Vesicle-associated urokinase plasminogen activator promotes invasion in prostate cancer cell lines. Clin Exp Metastasis 18:163–170
Dolo V, D’Ascenzo S, Giusti I et al (2005) Shedding of membrane vesicles by tumor and endothelial cells. Ital J Anat Embryol 110:127–133
Moisa A, Fritz P, Eck A et al (2007) Growth/adhesion-regulatory tissue lectin galectin-3: stromal presence but not cytoplasmic/nuclear expression in tumor cells as a negative prognostic factor in breast cancer. Anticancer Res 27:2131–2139
Duca L, Floquet N, Alix AJP et al (2004) Elastin as a matrikine. Crit Rev Oncol Hematol 49:235–244
Acknowledgments
Simon Toupance is supported by a grant from the Ministry of Research. This work is supported by the Lions’s Clubs of Soissons, Villers-Cotterets and Crépy en Valois, by ACI Cancéropôle Grand Est 2007-2009 and by a grant from the CRP Santé of Luxembourg.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Philippe Birembaut and Laurent Debelle have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Toupance, S., Brassart, B., Rabenoelina, F. et al. Elastin-derived peptides increase invasive capacities of lung cancer cells by post-transcriptional regulation of MMP-2 and uPA. Clin Exp Metastasis 29, 511–522 (2012). https://doi.org/10.1007/s10585-012-9467-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-012-9467-3