Summary
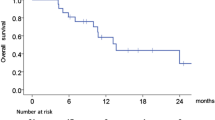
Aim: to determine whether EMD 1201081, a TLR9 agonist, added to cetuximab had antitumor activity in second-line recurrent/metastatic squamous cell carcinoma of the head and neck (R/M SCCHN) Methods: this was a phase 2, open-label, randomized trial of EMD 1201081 0.32 mg/kg subcutaneously weekly plus cetuximab (combination) vs cetuximab monotherapy (control) in cetuximab-naïve patients with R/M SCCHN who progressed on 1 cytotoxic regimen. Crossover to combination was permitted after progression Results: objective response rate in both arms was 5.7 % (95 % CI 1.2–15.7 %) by independent assessment. Disease control was 37.7 % for patients on combination (24.8–52.1 %) and 43.4 % on control (29.8–57.7 %). Neither independent nor investigator assessments showed significant differences between study arms. Median progression-free survival was 1.5 months (1.3–2.6) for patients on combination, and 1.9 months (1.5–2.9) on control.
The most frequent adverse events in the combination arm were rash (29.6 %), acneiform dermatitis (22.2 %), and injection site reactions (20.4 %). Grade 3/4 dyspnea and hypokalemia were more frequent with cetuximab monotherapy (7.5 % and 5.7 % vs 1.9 % each, respectively), and grade 3/4 respiratory failure and disease progression were more frequent with combination (5.6 % each vs 1.9 % each) Conclusion: EMD 1201081 was well tolerated combined with cetuximab, but there was no incremental clinical efficacy.


Similar content being viewed by others
References
Ahmed SM, Cohen EEW (2007) Treatment of squamous cell carcinoma of the head and neck in the metastatic and refractory settings: advances in chemotherapy and the emergence of small molecule epidermal growth factor receptor kinase inhibitors. Curr Cancer Drug Targets 7:666–73
Globocan 2012: http://globocan.iarc.fr/old/bar_sex_site_prev.asp?selection=11100&selection=13010&selection=21030&title=Larynx%2C+Lip%2C+oral+cavity%2C+Other+pharynx&statistic=1&populations=6&window=1&grid=1&info=1&color1=5&color1e=&color2=4&color2e=&submit=%C2%A0Execute; downloaded 13 April 2014
Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH (2004) Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 350:1945–1952. doi:10.1056/NEJMoa032641
Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB (2004) Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 350:1937–1944. doi:10.1056/NEJMoa032646
Zimmermann M, Zouhair A, Azria D, Ozsahin M (2006) The epidermal growth factor receptor (EGFR) in head and neck cancer: its role and treatment implications. Radiat Oncol 1:11. doi:10.1186/1748-717X-1-11 DOI:10.1186%2F1748-717X-1-11#pmc_ext
Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK (2010) Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 11(1):21–28
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116–11127. doi:10.1056/NEJMoa0802656
Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F (2007) Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol 25:2171–2177
Damiano V, Caputo R, Bianco R, D’Armiento FP, Leonardi A, De Placido S (2006) Novel toll-like receptor 9 agonist induces epidermal growth factor receptor (EGFR) inhibition and synergistic antitumor activity with EGFR inhibitors. Clin Cancer Res 12:577–583
Takeda K, Akira S (2005) Toll-like receptors in innate immunity. Int Immunol 17(1):1–14
Wickelgren I (2006) Targeting the tolls. Science 312:184–187
Wang D, Li Y, Yu D, Song SS, Kandimalla ER, Agrawal S (2004) Immunopharmacological and antitumor effects of second-generation immunomodulatory oligonucleotides containing synthetic CpR motifs. Int J Oncol 24:901–908
Damiano V, Caputo R, Garofalo S, Bianco R, Rosa R, Merola G et al (2007) TLR9 agonist acts by different mechanisms synergizing with bevacizumab in sensitive and cetuximab-resistant colon cancer xenografts. PNAS 104(30):12468–12473
Rosa R, Melisi D, Damiano V, Bianco R, Garofalo S, Gelardi T et al (2011) Toll-like receptor 9 agonist IMO cooperates with cetuximab in K-ras mutant colorectal and pancreatic cancers. Clin Cancer Res 17(20):6531–6541. doi:10.1158/1078-0432.CCR-10-3376
Moore DJ, Hwang J, McGreivy J, Park S, Malik S, Martin RR et al (2005) Phase I trial of escalating doses of the TLR9 agonist HYB2055 in patients with advanced solid tumors. J Clin Oncol 23(16S):2503
Kuzel T, Dutcher J, Ebbinghaus S, Gordon, M, Grubbs, S, Khan, K, et al. (2009) A phase 2 multicenter, randomized, open-label study of two dose levels of IMO-2055 in patients with metastatic or recurrent renal cell carcinoma. Poster presented at the 8th International Kidney Cancer Symposium, Sep 25–26 2009, Chicago, IL, USA
Malik S, Hwang J, Cotarla I, Sullivan T, Karr R, Marshall J et al (2007) Initial phase 1 results of gemcitabine, carboplatin and IMO-2055, a toll like receptor 9 (TLR9) agonist, in patients (pts) with advanced solid tumors. J Thor Oncol 2(8):S726–S727. doi:10.1097/01.JTO.0000284088.13204.12
Smith DA, Conkling P, Richards DA, Flores MR, Nemunaitis JJ, Boyd TE et al. (2012) Efficacy and safety of IMO-2055, a novel T LR9 agonist, in combination with erlotinib (E) and bevacizumab (bev) in patients (pts) with advanced or metastatic non-small cell lung cancer (NSCLC) who have progressed following prior chemotherapy. J Clin Oncol 30, (suppl; abstr e18047)
Machiels JP, Kaminsky MC, Keller U, Brümmendorf TH, Goddemeier T, Forssmann U, et al.. (2013) Phase Ib trial of IMO-2055 in combination with 5-FU, cisplatin and cetuximab as first-line palliative treatment in patients with recurrent/metastatic squamous cell carcinoma of the head and neck. Invest New Drugs: Feb 10 [Epub ahead of print]
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al (2000) New Guidelines to evaluate the response to treatment in solid tumors (RECIST Guidelines). J Natl Cancer Inst 92:205–216. doi:10.1093/jnci/92.3.205
CTCAE (Common Terminology Criteria for Adverse Events) (2003), Version 3.0, 12 December 2003
Manegold C, van Zandwijk N, Szczesna A, Zatloukal P, Au JS, Blasinska-Morawiec M et al (2012) A phase III randomized study of gemcitabine and cisplatin with or without PF-3512676 (TLR9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol 23(1):72–77. doi:10.1093/annonc/mdr030
Hirsh V, Boyer M, Rosell R, Rosell R, Middleton G, Eberhardt WE, et al. (2008) Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 as first line treatment of advanced non-small cell lung cancer (NSCLC). J Clin Oncol 26: (May 20 suppl; abstr 8016)
Manegold C, Gravenor D, Woytowitz D, Mezger J, Hirsh V, Albert G et al (2008) Randomized phase II trial of a toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage Non–small-cell lung cancer. J Clin Oncol 26(24):3979–3986
Hirsh V, Paz-Ares L, Boyer M, Rosell R, Middleton G, Eberhardt WE et al (2011) Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 29(19):2667–2674. doi:10.1200/JCO.2010.32.8971
Rodriguez-Torres M, Ghalib RH, Gordon SC, E Lawitz, Patel K, Pruitt R, et al. (2010) IMO-2125, A TLR9 Agonist, Induces Immune Responses Which Correlate With Reductions In Viral Load In Null Responder HCV Patients. Hepatology, 52 (4 - Suppl), October abstr 33, 336A
Dynavax–FDA advisory committee briefing materials provided prior to the 15 November 2012 meeting of the Vaccines and Related Biological Products Advisory Committee
Acknowledgments
This study was sponsored by Merck-Serono SA – Geneva, Switzerland. Writing support was provided by Idera pharmaceuticals.
Ethical standards
The authors state that the experiments described in this manuscript comply with the current laws of the country in which they were performed.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Ruzsa, A., Sen, M., Evans, M. et al. Phase 2, open-label, 1:1 randomized controlled trial exploring the efficacy of EMD 1201081 in combination with cetuximab in second-line cetuximab-naïve patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN). Invest New Drugs 32, 1278–1284 (2014). https://doi.org/10.1007/s10637-014-0117-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0117-2