Summary
Purpose
To determine the maximum tolerated dose (MTD) of the combination of linsitinib (OSI-906), a dual inhibitor of IGFR and IR tyrosine kinase activity, and everolimus as treatment for patients with refractory metastatic colorectal cancer (mCRC).
Methods
Eligible adult patients with refractory mCRC, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate end-organ function received escalating doses of OSI-906 and everolimus in a 3 + 3 design. Treatment continued until disease progression or unacceptable toxicity, with response evaluations every 8 weeks.
Results
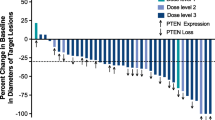
Eighteen patients with metastatic CRC were treated. There were no dose-limiting toxicities (DLTs) in the first dose level (DL, OSI-906 50 mg BID; everolimus 5 mg QD). At DL2 (OSI-906 100 mg BID; everolimus 10 mg QD, n =6), three patients had DLTs considered related to everolimus (grade 3 mucositis, 2; grade 3 thrombocytopenia, 1). An amendment introduced DL2a (OSI-906 100 mg BID; everolimus 5 mg QD, n =5); DLTs were seen in two patients (one patient each: grade 3 thrombocytopenia with bleeding; inability to receive 75 % of doses due to neutropenia/thrombocytopenia). DL1 was the MTD; a total of 7 patients were treated at this dose. Common adverse events across all DLs included grade 1/2 fatigue (50 %) and anorexia (50 %). There were no objective responses to treatment; median time of study treatment was 7.6 weeks (range: 3.9–53 weeks).
Conclusions
The MTD of OSI-906 and everolimus was 50 mg BID and 5 mg QD, respectively. No indications of clinical activity were observed in refractory mCRC patients.


Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics. CA: A Cancer Journal for Clinicians 62:10–29
Van Cutsem E, Peeters M, Siena S et al (2007) Open-Label Phase III Trial of Panitumumab Plus Best Supportive Care Compared With Best Supportive Care Alone in Patients With Chemotherapy-Refractory Metastatic Colorectal Cancer. J Clin Oncol 25:1658–1664
Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-Kinase-AKT pathway in human cancer. Nat Rev Cancer 2:489–501
Altomare I, Bendell JC, Bullock KE et al (2011) A Phase II Trial of Bevacizumab plus Everolimus for Patients with Refractory Metastatic Colorectal Cancer. The Oncologist 16:1131–1137
Evans T, Lindsay CR, Chan E et al (2010) Phase I dose-escalation study of continuous oral dosing of OSI-906, a dual tyrosine kinase inhibitor of insulin-like growth factor-1 receptor (IGF-1R) and insulin receptor (IR), in patients with advanced solid tumors. ASCO Meeting Abstracts 28:2531
Baserga R (1999) The IGF-I Receptor in Cancer Research. Experimental Cell Research 253:1–6
Baserga R, Peruzzi F, Reiss K (2003) The IGF-1 receptor in cancer biology. International Journal of Cancer 107:873–877
Kurmasheva RT, Houghton PJ (2006) IGF-I mediated survival pathways in normal and malignant cells. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1766:1–22
Tao Y, Pinzi V, Bourhis J, Deutsch E (2007) Mechanisms of Disease: signaling of the insulin-like growth factor 1 receptor pathway [mdash]therapeutic perspectives in cancer. Nat Clin Prac Oncol 4:591–602
Baserga R (2005) The insulin-like growth factor-I receptor as a target for cancer therapy. Expert Opinion on Therapeutic Targets 9:753–768
O’Reilly KE, Rojo F, She Q-B et al (2006) mTOR Inhibition Induces Upstream Receptor Tyrosine Kinase Signaling and Activates Akt. Cancer Research 66:1500–1508
Wan X, Harkavy B, Shen N et al (2006) Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26:1932–1940
Declaration of Helsinki. In. www.wma.net/e/ethicsunit/helsinki.htm: World Medical Association, Ethics Unit 2007.
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). European Journal of Cancer 45:228–247
Storer BE (1989) Design and analysis of phase I clinical trials. Biometrics 45:925–937
NCI Common Terminology Criteria for Adverse Events (CTCAE) v.4. In. http://evs.nci.gov/ftp1/CTCAE/about/html: National Cancer Institute 2010.
Fuchs CS, Tabernero JM, Hwang J et al. Multicenter phase II study of RAD001 in patients with chemotherapy-refractory metastatic colorectal cancer (mCRC). 2009 Gastrointestinal Cancers Symposium 2009; Abstr #446.
Baselga J, Campone M, Piccart M et al (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520–529
Wolpin BM, Wei EK, Ng K et al (2008) Prediagnostic Plasma Folate and the Risk of Death in Patients With Colorectal Cancer. Journal of Clinical Oncology 26:3222–3228
Watkins DJ, Tabernero J, Schmoll H et al (2011) A randomized phase II/III study of the anti-IGF-1R antibody MK-0646 (dalotuzumab) in combination with cetuximab (Cx) and irinotecan (Ir) in the treatment of chemorefractory metastatic colorectal cancer (mCRC) with wild-type (wt) KRAS status. ASCO Meeting Abstracts 29:3501
Sathyanarayanan S, Ayers M, Cunningham D et al (2012) Activity of the anti-IGF-1R antibody dalotuzumab (MK-0646) in KRAS-mutant colorectal cancer: Preclinical and clinical data. ASCO Meeting Abstracts 30:3587
Eng C, Van Cutsem E, Nowara E et al (2011) A randomized, phase Ib/II trial of rilotumumab (AMG 102; ril) or ganitumab (AMG 479; gan) with panitumumab (pmab) versus pmab alone in patients (pts) with wild-type (WT) KRAS metastatic colorectal cancer (mCRC): Primary and biomarker analyses. ASCO Meeting Abstracts 29:3500
Sun S-Y, Rosenberg LM, Wang X et al (2005) Activation of Akt and eIF4E Survival Pathways by Rapamycin-Mediated Mammalian Target of Rapamycin Inhibition. Cancer Research 65:7052–7058
Tamburini J, Chapuis N, Bardet V et al (2008) Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood 111:379–382
Quek R, Wang Q, Morgan JA et al (2011) Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res 17:871–879
Di Cosimo S, Bendell JC, Cervantes-Ruiperez A et al (2010) A phase I study of the oral mTOR inhibitor ridaforolimus (RIDA) in combination with the IGF-1R antibody dalotozumab (DALO) in patients (pts) with advanced solid tumors. ASCO Meeting Abstracts 28:3008
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
By Novartis Pharmaceuticals and OSI Pharmaceuticals, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bendell, J.C., Jones, S.F., Hart, L. et al. A phase Ib study of linsitinib (OSI-906), a dual inhibitor of IGF-1R and IR tyrosine kinase, in combination with everolimus as treatment for patients with refractory metastatic colorectal cancer. Invest New Drugs 33, 187–193 (2015). https://doi.org/10.1007/s10637-014-0177-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0177-3