Abstract
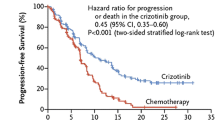
The anaplastic lymphoma kinase (ALK) fusion gene is a key oncogenic driver in a subset of patients with advanced non-small cell lung cancer (NSCLC). Oncogenic fusion genes, including echinoderm microtubule-associated protein-like 4 (EML4) and ALK, have been detected in approximately 2–7 % of NSCLC patients. Fluorescence in situ hybridization (FISH) is the recommended method for detecting ALK gene rearrangement. EML4–ALK fusion genes define a molecular subset of NSCLC with distinct clinical characteristic (lung adenocarcinoma, never or former smoker, usually mutually exclusive with EGFR mutations). Crizotinib (PF-02341066) is an orally bioavailable, ATP-competitive, small molecule inhibitor of both the receptor tyrosine kinases ALK and c-MET (hepatocyte growth factor receptor). Crizotinib has been shown to yield important clinical benefit such as objective response rate, progression-free survival (PFS), and anticipated improvements in quality of life when used in pretreated patients with advanced NSCLC harboring EML4–ALK gene rearrangement. Preliminary phase II data suggested that crizotinib is safe and well tolerated with rapid and robust antitumor activity. A phase III randomized trial in a second-line setting showed response rate and PFS (primary study endpoint) advantage for crizotinib as compared to second-line chemotherapy. Treatment-related adverse events, predominantly restricted to the gastrointestinal and visual systems, are generally self-limiting or easily managed. Crizotinib is a new standard of care for patients with advanced, ALK-positive, NSCLC. In this review, we will discuss the discovery of ALK rearrangements, the clinical epidemiology of lung cancer driven by ALK, the clinical data for ALK-targeted therapy in NSCLC, and ongoing ALK inhibitor-based clinical trials.

Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F et al (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Shiller JR, Harrington D, Belani CP et al (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92–98
Sandler A, Gray R, Perry MC et al (2006) Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Lynch TJ, Bell DW, Sordella R et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350:2129–2139
Paez JG, Jänne PA, Lee JC et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500
Mok TS, Wu YL, Thongprasert S et al (2009) Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Rosell R, Carcereny E, Gervais R et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13(3):239–246
Soda M, Choi YL, Enomoto M et al (2007) Identification of the transforming EML4–ALK fusion gene in non-small cell lung cancer. Nature 448:561–566
Soda M, Takada S, Takeuchi K et al (2008) A mouse model for EML4–ALK-positive lung cancer. Proc Natl Acad Sci 105:19893–19897
Wasik MA et al (2009) Anaplastic lymphoma kinase (ALK)-induced malignancies: novel mechanisms of cell transformation and potential therapeutic approaches. Semin Oncol 36(2 Suppl 1):S27–S35
Morris SW et al (1994) Fusion of a kinase gene, ALK to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 263(5151):1281–1284
Houtman SH et al (2007) Echinoderm microtubule-associated protein like protein 4, a member of the echinoderm microtubule-associated protein family, stabilizes microtubules. Neuroscience 144(4):1373–1382
Pollmann M, Parwaresch R, Adam-Klages S (2006) Human EML4, a novel member of the EMAP family, is essential for microtubule formation. Exp Cell Res 312:3241–3251
Amin HM, Lai R (2007) Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood 110(7):2259–2267
Chiarle R, Voena C, Ambrogio C et al (2008) The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer 8:11–23
Sasaki T, Rodig SJ, Chirieac LR et al (2010) The biology and treatment of EML4–ALK non-small cell lung cancer. Eur J Cancer 46:1773–1780
Choi YL, Takeuchi K, Soda M et al (2008) Identification of novel isoforms of the EML4–ALK transforming gene in non-small cell lung cancer. Cancer Res 68:4971–4976
Koivunen JP, Mermel C, Zejnullahu K et al (2008) EML4–ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 14:4275–4283
Takeuchi K, Choi YL, Soda M et al (2008) Multiplex reverse transcription-PCR screening for EML4–ALK fusion transcripts. Clin Cancer Res 14:6618–6624
Takeuchi K, Choi YL, Togashi Y et al (2009) KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res 15:3143–3149
Inamura K, Takeuchi K, Togashi Y et al (2008) EML4–ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol 3:13–17
Perner S, Wagner PL, Demichelis F et al (2008) EML4–ALK fusion lung cancer: a rare acquired event. Neoplasia 10:298–302
Shinmura K, Kageyama S, Tao H et al (2008) EML4–ALK fusion transcripts, but no NPM-, TPM3-, CLTC-, ATIC-, or TFG-ALK fusion transcripts, in non-small cell lung carcinomas. Lung Cancer 61:163–169
Martelli MP, Sozzi G, Hernandez L et al (2009) EML4–ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol 174:661–670
Wong DW, Leung EL, So KK et al (2009) The EML4–ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 115:1723–1733
Shaw AT, Yeap BY, Mino-Kenudson M et al (2009) Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4–ALK. J Clin Oncol 27:4247–4253
Takahashi T, Sonobe M, Kobayashi M et al (2010) Clinicopathologic features of non-small-cell lung cancer with EML4–ALK fusion gene. Ann Surg Oncol 17:889–897
Inamura K, Takeuchi K, Togashi Y et al (2009) EML4–ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol 22:508–515
Horn L, Pao W (2009) EML4–ALK: honing in on a new target in non-small cell lung cancer. J Clin Oncol 27:4232–4235
Rikova K, Guo A, Zeng Q et al (2007) Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 131:1190–1230
Hernandez L, Pinyol M, Hernandez S et al (1999) TRK-fused gene (TFG) is a new partner of ALK in anaplastic large cell lymphoma producing two structurally different TFG-ALK translocations. Blood 94(9):3265–3268
Rodig SJ, Mino-Kenudson M, Dacic S et al (2009) Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res 15:5216–5223
Camidge DR, Kono SA, Flacco A et al (2010) Optimizing the detection of lung cancer patients harboring anaplastic lymphoma kinase (ALK) gene rearrangements potentially suitable for ALK inhibitor treatment. Clin Cancer Res 16:5581–5590
Yasuda H, Soejima K, Nakayama S et al (2011) Bronchoscopic microsampling is a useful complementary diagnostic tool for detecting lung cancer. Lung Cancer 72(1):32–38
Li T, Huang E, Desai S, et al (2012) Update on the large-scale screening of ALK fusion oncogene transcripts in archival NSCLC tumor specimens using multiplexed RT-PCR assays. Presented at ASCO 2012; abstract 7594
Jokoji R, Yamasaki T, Minami S et al (2010) Combination of morphological feature analysis and immunohistochemistry is useful for screening of EML4–ALK-positive lung adenocarcinoma. J Clin Pathol 63:1066–1070
Sakairi Y, Nakajima T, Yasufuku K et al (2010) EML4–ALK fusion gene assessment using metastatic lymph node samples obtained by endobronchial ultrasound guided transbronchial needle aspiration. Clin Cancer Res 16:4938–4945
Park HS, Lee JK, Kim DW et al (2012) Immunohistochemical screening for anaplastic lymphoma kinase (ALK) rearrangement in advanced non-small cell lung cancer patients. Lung Cancer 77:288–292
Boland JM, Erdogan S, Vasmatzis G et al (2009) Anaplastic lymphoma kinase immunoreactivity correlates with ALK gene rearrangement and transcriptional up-regulation in non-small cell lung carcinomas. Hum Pathol 40:1152–1158
Zhang X, Zhang S, Yang X et al (2010) Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer 9:188
Yung-Jue B (2011) The potential for crizotinib in non-small cell lung cancer: a perspective review. The Adv Med Oncol 3(6):279–291
Christensen JG, Zou HY, Arango ME et al (2007) Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther 6:3314–3322
Zou HY et al (2007) An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res 67:4408–4417
Li R, Morris SW (2008) Development of anaplastic lymphoma kinase (ALK) small-molecule inhibitors for cancer therapy. Med Res Rev 28:372–412
Galkin AV et al (2007) Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM-ALK. Proc Natl Acad Sci USA 104(1):270–275
Shakespeare WC, et al (2009) Discovery of potent and selective orally active inhibitors of anaplastic lymphoma kinase (ALK). Proc Am Assoc Cancer Res 50 Abstract 3738
Rivera VM, et al (2010) Efficacy and pharmacodynamic analysis of AP26113, a potent and selective orally active inhibitor of anaplastic lymphoma kinase (ALK). Proc Am Assoc Cancer Res 51 Abstract 3623
Sakamoto H, Tsukaguchi T, Hiroshima S et al (2011) CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 19:679–690
Cheng M, Quail MR, Gingrich DE et al (2012) CEP-28122, a highly potent and selective orally active inhibitor of anaplastic lymphoma kinase with antitumor activity in experimental models of human cancers. Mol Cancer Ther 11(3):670–679
Lovly CM, et al (2010) Preclinical development of a selective, potent small molecule ALK inhibitor. Proc. Am. Assoc. Cancer Res 51 Abstract 1788
Kruczynski A et al (2009) Antitumor activity of pyridoisoquinoline derivatives F91873 and F91874, novel multikinase inhibitors with activity against the anaplastic lymphoma kinase. Anti-Cancer Drugs 20(5):364–372
Fancelli D et al (2006) 1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazoles: identification of a potent aurora kinase inhibitor with a favorable antitumor kinase inhibition profile. J Med Chem 49(24):7247–7251
Sabbatini P et al (2009) GSK1838705A inhibits the insulin-like growth factor-1 receptor and anaplastic lymphoma kinase and shows antitumor activity in experimental models of human cancers. Mol Cancer Ther 8(10):2811–2820
Chen Z, Sasaki T, Tan X et al (2010) Inhibition of ALK, PI3K/MEK, and HSP90 in murine lung adenocarcinoma induced by EML4–ALK fusion oncogene. Cancer Res 70:9827–9836
Lovly CM, Heuckmann JM, de Stanchina E et al (2011) Insights into ALK-driven cancers revealed through development of novel ALK tyrosine kinase inhibitors. Cancer Res 71:4920–4931
Garcia-Echeverria C, Kanazawa T, Kawahara E et al (2005) 2,4-Pyrimidinediamines useful in the treatment of neoplastic disease, inflammatory and immune system disorders. Novartis AG, Novartis Pharma
Ardini E, Menichincheri M, De Ponti C et al (2009) Medical characterization of NMS-E628, a small molecule inhibitor of anaplastic lymphoma kinase with antitumor efficacy in ALK-dependent lymphoma and non-small cell lung cancer models. Mol Cancer Ther 8(Suppl 1):A243
Jake Slavish P, Jiang Q, Cui X et al (2009) Design and synthesis of a novel tyrosine kinase inhibitor template. Bioorg Med Chem 17:3308–3316
Milkiewicz KL, Weinberg LR, Albom MS et al (2010) Synthesis and structure–activity relationships of 1,2,3,4-tetrahydropyrido[2,3-b]pyrazines as potent and selective inhibitors of the anaplastic lymphoma kinase. Bioorg Med Chem 18:4351–4362
Okamoto M, Kojima H, Saito N et al (2011) Virtual screening and further development of novel ALK inhibitors. Bioorg Med Chem 19:3086–3095
Sequist LV, Gettinger S, Senzer NN et al (2010) Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol 28:4953–4960
Kwak EL, Camidge DR, Clark J et al (2009) Clinical activity observed in a phase I dose escalation trial of an oral c-MET and ALK inhibitor, PF-02341066. J Clin Oncol 27(Suppl):148s
Kwak EL, Bang YJ, Camidge DR et al (2010) Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363:1693–1703
Camidge DR, Bang Y, Kwak EL, et al (2011) Progression-free survival (PFS) from a phase I study of crizotinib (PF-02341066) in patients with ALK-positive non-small cell lung cancer (NSCLC). Journal of Clinical Oncology, 2011, ASCO Annual Meeting Proceedings (Post-Meeting Edition). Vol 29, No 15_suppl (May 20 Supplement), 2011: 2501
Ou SI, Salgia R, Clark J, et al. (2010) Comparison of crizotinib (PF-02341066) pharmacokinetics between Asian and non-Asian patients with advanced malignancies. Presented at the 4th Asia Pacific Lung Cancer Conference (APLCC) Seoul, South Korea, 2–4 December 2010.
Camidge DR, Bang Y-B, Kwak EL et al (2012) Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 13:1011–1019
Weickhardt AJ, Rothman MS, Salian-Mehta S (2012) Rapid-onset hypogonadism secondary to crizotinib use in men with metastatic nonsmall cell lung cancer. Cancer 118(21):5302–5309
Shaw AT, Yeap BY, Solomon BJ et al (2011) Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 12(11):1004–1012
Kim DW, Ahn M-J, Shi Y, et al. (2012) Updated results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC). Presented at the 2012 ESMO; abstract 1230P
Shaw AT. Phase III trial shows crizotinib superior to single-agent chemotherapy for ALK-positive advanced NSCLC. Presented at ESMO 2012, abstract 2862
Ou S-HI, Govindan R, Eaton KD, et at (2012) Phase I/II dose-finding study of crizotinib (CRIZ) in combination with erlotinib (E) in patients (pts) with advanced non-small cell lung cancer (NSCLC). Presented at the 2012 ESMO; abstract 1291P
Camidge DR, Kono SA, Lu X et al (2011) Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol 6:774–780
Takezawa K, Okamoto I, Okamoto W et al (2011) Thymidylate synthase as a determinant of pemetrexed sensitivity in non-small cell lung cancer. Br J Cancer 104:1594–1601
Takeda M, Okamoto I, Sakai K, et al (2011) Successful long term treatment with pemetrexed of NSCLC associated with EML4–ALK and low thymidylate synthase expression. Clinical Lung Cancer, vol XX, No X
Bergethon K, Shaw AT, Ou SI et al (2012) ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 30:863–870
Ou SI, Camidge DR, Engelman JA, et al (2012) Clinical activity of crizotinib in patients with advanced non-small cell lung cancer harboring ROS1 gene rearrangement. Presented at ESMO 2012; abstract 1191PD
Camidge DR, Doebele RC et al (2012) Treating ALK-positive lung cancer—early successes and future challenges. Nat Rev Clin Oncol 9(5):268–277
Doebele RC, Pilling AB, Aisner DL et al (2012) Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 18(5):1472–1482
Katayama R, Shaw AT, Khan TM et al (2012) Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med 4(120):120ra17
Choi YL, Soda M, Yamashita Y et al (2010) EML4–ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 363:1734–1739
Doebele RC, Aisner DL, Le AT, et al (2012) Analysis of resistance mechanisms to ALK kinase inhibitors in ALK+ NSCLC patients. J Clin Oncol 30 (suppl; abstr 7504)
Nishio M, Kiura K, Nakagawa K, et al (2012) A phase I/II study of ALK inhibitor CH5424802 in patients with ALK-positive NSCLC; safety and efficacy interim results of the phase II portion. Presented at ESMO 2012; abstract 441O
Katayama R, Khan TM, Benes C et al (2011) Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4–ALK. Medical Science 108(no. 18):7535–7540
Sequist LV, Janne PA, Sweeney J, et al. (2007) Phase 1/2 trial of the novel Hsp90 inhibitor, IPI-504, in patients with relapsed and/or refractory stage IIIb or stage IV non-small cell lung cancer (NSCLC) stratified by EGFR mutation status. AACR-NCIEORTC International Conference on Molecular Targets and Cancer Therapeutics, October 22–26, San Francisco, CA.
Zhang S, Wang F, Keats J et al (2011) Crizotinib-resistant mutants of EML4–ALK identified through an accelerated mutagenesis screen. Chem Biol Drug Des 78:999–1005
Zhang S, Wang F, Keats J, et al (2010) AP26113, a potent ALK inhibitor, overcomes mutations in EML4–ALK that confer resistance to PF-02341066 (PF1066). Presented at the 101st American Association for Cancer Research Annual Meeting, 2010, Abstract LB-298
Felip E, Carcenery E, Barlesi F, et al (2012) Phase II activity of the Hsp90 inhibitor AUY922 in patients with ALK rearranged (ALK+) or EGFR-mutated advanced non-small cell lung cancer (NSCLC). Presented at ESMO 2012. Abstract 438O
Shaw AT, Camidge DR, Felip E, et al (2012) Results of a first-in-human phase I study of the ALK inhibitor LDK378 in advanced solid tumors. Presented at ESMO 2012; abstract 440O
Conflict of interest
Gridelli C. received honoraria as speaker bureau member and is an ordinary board member by Roche and Eli Lilly.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Casaluce, F., Sgambato, A., Maione, P. et al. ALK inhibitors: a new targeted therapy in the treatment of advanced NSCLC. Targ Oncol 8, 55–67 (2013). https://doi.org/10.1007/s11523-012-0250-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-012-0250-9