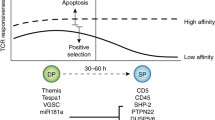
Abstract
T cell recognition of antigen is a crucial aspect of the adaptive immune response. One of the most common means of pathogen immune evasion is mutation of T cell epitopes. T cell recognition of such ligands can result in a variety of outcomes including activation, apoptosis and anergy. The ability of a given T cell to respond to a specific peptide–MHC ligand is regulated by a number of factors, including the affinity, on- and off-rates and half-life of the TCR–peptide–MHC interaction. Interaction of T cells with low-potency ligands results in unique signaling patterns and requires engagement with a larger number of T cell receptors than agonist ligands. This review will address these aspects of T cell interaction with weak ligands and the ways in which these ligands have been utilized therapeutically.



Similar content being viewed by others
References
Evavold BD, Allen PM. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science. 1991;252:1308–10.
De Magistris MT, Alexander J, Coggeshall M, Altman A, Gaeta FCA, Grey HM, Sette A. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992;68:625–34.
Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156–9.
Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–22.
Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, Germain RN. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 1995;267:515–8.
Huppa J, Axmann BM, Mörtelmaier MA, Lillemeier BF, Newell EW, Brameshuber M, Klein LO, Schütz GJ, Davis MM. TCR–peptide–MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 2010;463:963–7.
Huang J, Zarnitsyna VI, Liu B, Edwards LJ, Jiang N, Evavold BD, Zhu C. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 2010;464:932–6.
Bertoletti A, Costanzo A, Chisari FV, Levrero M, Artini M, Sette A, Penna A, Giuberti T, Fiaccadori F, Ferrari C. Cytotoxic T lymphocyte response to a wild type hepatitis B virus epitope in patients chronically infected by variant viruses carrying substitutions within the epitope. J Exp Med. 1994;180:933–43.
Bertoletti A, Sette A, Chisari FV, Penna A, Levrero M, De Carli M, Fiaccadori F, Ferrari C. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T cells. Nature. 1994;369:407–10.
Klenerman P, Rowland-Jones S, McAdam S, Edwards J, Daenke S, Lalloo D, Koppe B, Rosenberg W, Boyd D, Edwards A, Giangrande P, Phillips RE, McMichael AJ. Cytotoxic T-cell activity antagonized by naturally occurring HIV-1 Gag variants. Nature. 1994;369:403–10.
Johanns TM, Ertelt JM, Lai JC, Rowe JH, Avant RA, Way SS. Naturally occurring altered peptide ligands control Salmonella-specific CD4+ T cell proliferation, IFN-gamma production, and protective potency. J Immunol. 2010;184:869–76.
Gilbert SC, Plebanski M, Gupta S, Morris J, Cox M, Aidoo M, Kwiatkowski D, Greenwood BM, Whittle HC, Hill AV. Association of malaria parasite population structure, HLA, and immunological antagonism. Science. 1998;279:1173–7.
Domingo E, Holland JJ. RNA virus mutations and fitness for survival. Annu Rev Microbiol. 1997;51:151–78.
Kubota R, Soldan SS, Martin R, Jacobson S. An altered peptide ligand antagonizes antigen-specific T cells of patients with human T lymphotropic virus type I-associated neurological disease. J Immunol. 2000;164:5192–8.
Frasca L, Del Porto P, Tuosto L, Marinari B, Scotta C, Carbonari M, Nicosia A, Piccolella E. Hypervariable region 1 variants act as TCR antagonists for hepatitis C virus-specific CD4+ T cells. J Immunol. 1999;163:650–8.
Ciurea A, Hunziker L, Martinic MM, Oxenius A, Hengartner H, Zinkernagel RM. CD4+ T-cell-epitope escape mutant virus selected in vivo. Nat Med. 2001;7:795–800.
Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–62.
Lee EA, Flanagan KL, Minigo G, Reece WH, Bailey R, Pinder M, Hill AV, Plebanski M. Dimorphic Plasmodium falciparum merozoite surface protein-1 epitopes turn off memory T cells and interfere with T cell priming. Eur J Immunol. 2006;36:1168–78.
Sewell AK, Harcourt GC, Goulder PJ, Price DA, Phillips RE. Antagonism of cytotoxic T lymphocyte-mediated lysis by natural HIV-1 altered peptide ligands requires simultaneous presentation of agonist and antagonist peptides. Eur J Immunol. 1997;27:2323–9.
Purbhoo MA, Sewell AK, Klenerman P, Goulder PJ, Hilyard KL, Bell JI, Jakobsen BK, Phillips RE. Copresentation of natural HIV-1 agonist and antagonist ligands fails to induce the T cell receptor signaling cascade. Proc Natl Acad Sci USA. 1998;95:4527–32.
Puglielli MT, Zajac AJ, van der Most RG, Dzuris JL, Sette A, Altman JD, Ahmed R. In vivo selection of a lymphocytic choriomeningitis virus variant that affects recognition of the GP33–43 epitope by H-2Db but not H-2Kb. J Virol. 2001;75:5099–107.
Wang S, Buchli R, Schiller J, Gao J, VanGundy RS, Hildebrand WH, Eckels DD. Natural epitope variants of the hepatitis C virus impair cytotoxic T lymphocyte activity. World J Gastroenterol. 2010;16:1953–69.
Plebanski M, Lee EA, Hannan CM, Flanagan KL, Gilbert SC, Gravenor MB, Hill AV. Altered peptide ligands narrow the repertoire of cellular immune responses by interfering with T-cell priming. Nat Med. 1999;5:565–71.
Haanen JB, Wolkers MC, Kruisbeek AM, Schumacher TN. Selective expansion of cross-reactive CD8(+) memory T cells by viral variants. J Exp Med. 1999;190:1319–28.
Haribhai D. Functional reprogramming of the primary immune response by t cell receptor antagonism. J Exp Med. 2004;200:1371–82.
Bouhdoud L, Villain P, Merzouki A, Arella M, Couture C. T-cell receptor-mediated anergy of a human immunodeficiency virus (HIV) gp120-specific CD4(+) cytotoxic T-cell clone, induced by a natural HIV type 1 variant peptide. J Virol. 2000;74:2121–30.
Ream RM, Sun J, Braciale TJ. Stimulation of naive CD8+ T cells by a variant viral epitope induces activation and enhanced apoptosis. J Immunol. 2010;184:2401–9.
Schnell FJ, Alberts-Grill N, Evavold BD. CD8+ T cell responses to a viral escape mutant epitope: active suppression via altered SHP-1 activity. J Immunol. 2009;182:1829–35.
Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NR. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–20.
Rosette C, Werlen G, Daniels MA, Holman PO, Alam SM, Travers PJ, Gascoigne NR, Palmer E, Jameson SC. The impact of duration versus extent of TCR occupancy on T cell activation: a revision of the kinetic proofreading model. Immunity. 2001;15:59–70.
Chesla SE, Selvaraj P, Zhu C. Measuring two-dimensional receptor-ligand binding kinetics by micropipette. Biophys J. 1998;75:1553–72.
Huang J, Edwards LJ, Evavold BD, Zhu C. Kinetics of MHC-CD8 interaction at the T cell membrane. J Immunol. 2007;179:7653–62.
Sabatino JJ, Huang J, Zhu C, Evavold BD. High prevalence of low affinity peptide-MHC II tetramer-negative effectors during polyclonal CD4+ T cell responses. J Exp Med. 2011;208:81–90.
Jiang N, Huang J, Edwards LJ, Liu B, Zhang Y, Beal CD, Evavold BD, Zhu C. Two-stage cooperative T cell receptor-peptide major histocompatibility complex-CD8 trimolecular interactions amplify antigen discrimination. Immunity. 2011;34:13–23.
Montixi C, Langlet C, Bernard AM, Thimonier J, Dubois C, Wurbel MA, Chauvin JP, Pierres M, He HT. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 1998;17:5334–48.
Smyth LA, Ardouin L, Williams O, Norton T, Tybulewicz V, Kioussis D. Inefficient clustering of tyrosine-phosphorylated proteins at the immunological synapse in response to an antagonist peptide. Eur J Immunol. 2002;32:3386–94.
Wulfing C, Rabinowitz JD, Beeson C, Sjaastad MD, McConnell HM, Davis MM. Kinetics and extent of T cell activation as measured with the calcium signal. J Exp Med. 1997;185:1815–25.
Sumen C. T cell receptor antagonism interferes with MHC clustering and integrin patterning during immunological synapse formation. J Cell Biol. 2004;166:579–90.
Ehrlich LI, Ebert PJ, Krummel MF, Weiss A, Davis MM. Dynamics of p56lck translocation to the T cell immunological synapse following agonist and antagonist stimulation. Immunity. 2002;17:809–22.
Huang J, Sugie K, La Face DM, Altman A, Grey HM. TCR antagonist peptides induce formation of APC-T cell conjugates and activate a Rac signaling pathway. Eur J Immunol. 2000;30:50–8.
Huang J, Tilly D, Altman A, Sugie K, Grey HM. T-cell receptor antagonists induce Vav phosphorylation by selective activation of Fyn kinase. Proc Natl Acad Sci USA. 2000;97:10923–9.
Edwards LJ, Evavold BD. A unique unresponsive CD4+ T cell phenotype post TCR antagonism. Cell Immunol. 2010;261:64–8.
Dittel BN, Stefanova I, Germain RN, Janeway CA Jr. Cross-antagonism of a T cell clone expressing two distinct T cell receptors. Immunity. 1999;11:289–98.
Kilgore NE, Carter JD, Lorenz U, Evavold BD. Cutting edge: dependence of TCR antagonism on Src homology 2 domain-containing protein tyrosine phosphatase activity. J Immunol. 2003;170:4891–5.
Somani AK, Bignon JS, Mills GB, Siminovitch KA, Branch DR. Src kinase activity is regulated by the SHP-1 protein-tyrosine phosphatase. J Biol Chem. 1997;272:21113–9.
Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–54.
Plas DR, Johnson R, Pingel JT, Matthews RJ, Dalton M, Roy G, Chan AC, Thomas ML. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 1996;272:1173–6.
Brockdorff J, Williams S, Couture C, Mustelin T. Dephosphorylation of ZAP-70 and inhibition of T cell activation by activated SHP1. Eur J Immunol. 1999;29:2539–50.
Jiao H, Berrada K, Yang W, Tabrizi M, Platanias LC, Yi T. Direct association with and dephosphorylation of Jak2 kinase by the SH2-domain-containing protein tyrosine phosphatase SHP-1. Mol Cell Biol. 1996;16:6985–92.
Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–38.
Cuevas B, Lu Y, Watt S, Kumar R, Zhang J, Siminovitch KA, Mills GB. SHP-1 regulates Lck-induced phosphatidylinositol 3-kinase phosphorylation and activity. J Biol Chem. 1999;274:27583–9.
Caron D, Savard PE, Doillon CJ, Olivier M, Shink E, Lussier JG, Faure RL. Protein tyrosine phosphatase inhibition induces anti-tumor activity: evidence of Cdk2/p27 kip1 and Cdk2/SHP-1 complex formation in human ovarian cancer cells. Cancer Lett. 2008;262:265–75.
Simoneau M, Boulanger J, Coulombe G, Renaud MA, Duchesne C, Rivard N. Activation of Cdk2 stimulates proteasome-dependent truncation of tyrosine phosphatase SHP-1 in human proliferating intestinal epithelial cells. J Biol Chem. 2008;283:25544–56.
Migone TS, Cacalano NA, Taylor N, Yi T, Waldmann TA, Johnston JA. Recruitment of SH2-containing protein tyrosine phosphatase SHP-1 to the interleukin 2 receptor; loss of SHP-1 expression in human T-lymphotropic virus type I-transformed T cells. Proc Natl Acad Sci USA. 1998;95:3845–50.
Zhang Z, Shen K, Lu W, Cole PA. The role of C-terminal tyrosine phosphorylation in the regulation of SHP-1 explored via expressed protein ligation. J Biol Chem. 2003;278:4668–74.
Liu Y, Kruhlak MJ, Hao JJ, Shaw S. Rapid T cell receptor-mediated SHP-1 S591 phosphorylation regulates SHP-1 cellular localization and phosphatase activity. J Leukoc Biol. 2007;82:742–51.
Wasserman HA, Beal CD, Zhang Y, Jiang N, Zhu C, Evavold BD. MHC variant peptide-mediated anergy of encephalitogenic T cells requires SHP-1. J Immunol. 2008;181:6843–9.
Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, Klein LO, Davis MM, Chen CZ. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–61.
Daniels MA, Schober SL, Hogquist KA, Jameson SC. Cutting edge: a test of the dominant negative signal model for TCR antagonism. J Immunol. 1999;162:3761–4.
Yang W, Grey HM. Study of the mechanism of TCR antagonism using dual-TCR-expressing T Cells. J Immunol. 2003;170:4532–8.
Robertson JM, Evavold BD. Cutting edge: dueling TCRs: peptide antagonism of CD4+ T cells with dual antigen specificities. J Immunol. 1999;163:1750–4.
McNeil LK, Evavold BD. TCR reserve: a novel principle of CD4 T cell activation by weak ligands. J Immunol. 2003;170:1224–30.
Jones DS, Reichardt P, Ford ML, Edwards LJ, Evavold BD. TCR antagonism by peptide requires high TCR expression. J Immunol. 2008;181:1760–6.
Wasserman HA, Evavold BD. Induction of anergy by antibody blockade of TCR in myelin oligodendrocyte glycoprotein-specific cells. J Immunol. 2008;180:7259–64.
Labrecque N, Whitfield LS, Obst R, Waltzinger C, Benoist C, Mathis D. How much TCR does a T cell need? Immunity. 2001;15:71–82.
Harding CV, Unanue ER. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990;346:574–6.
Demotz S, Grey HM, Sette A. The minimal number of class II MHC-antigen complexes needed for T cell activation. Science. 1990;249:1028–30.
Kimachi K, Croft M, Grey HM. The minimal number of antigen-major histocompatibility complex class II complexes required for activation of naive and primed T cells. Eur J Immunol. 1997;27:3310–7.
Liu GY, Fairchild PJ, Smith RM, Prowle JR, Kioussis D, Wraith DC. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 1995;3:407–15.
Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–40.
Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25:261–70.
Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–62.
Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–81.
Ji Q, Perchellet A, Goverman JM. Viral infection triggers central nervous system autoimmunity via activation of CD8+ T cells expressing dual TCRs. Nat Immunol. 2010;11:628–34.
Karin N, Mitchell DJ, Brocke S, Ling N, Steinman L. Reversal of experimental autoimmune encephalomyelitis by a soluble peptide variant of a myelin basic protein epitope: T cell receptor antagonism and reduction of interferon gamma and tumor necrosis factor alpha production. J Exp Med. 1994;180:2227–37.
Nicholson L, Nicholson B, Greer JM, Sobel RA, Lees MB, Kuchroo VK. An altered peptide ligand mediates immune deviation and prevents autoimmune encephalomyelitis. Immunity. 1995;3:397–405.
Brocke S, Gijbels K, Allegretta M, Ferber I, Piercy C, Blankenstein T, Martin R, Utz U, Karin N, Mitchell D, Veromaa T, Waisman A, Gaur A, Conlon P, Fairchild PJ, Wraith DC, O’Garra A, Fathman CG, Steinman L. Treatment of experimental encephalomyelitis with a peptide analogue of myelin basic protein. Nature. 1996;379:343–6.
Kirshner SL, Zisman E, Fridkin M, Sela M, Mozes E. Altered peptide ligands of a myasthenogenic epitope as modulators of specific T-cell responses. Scand J Immunol. 1996;44:512–21.
Faber-Elmann A, Paas-Rozner M, Sela M, Mozes E. Altered peptide ligands act as partial agonists by inhibiting phospholipase C activity induced by myasthenogenic T cell epitopes. Proc Natl Acad Sci USA. 1998;95:14320–5.
Ikagawa S, Matsushita S, Chen YZ, Ishikawa T, Nishimura Y. Single amino acid substitutions on a Japanese cedar pollen allergen (Cry j 1)-derived peptide induced alterations in human T cell responses and T cell receptor antagonism. J Allergy Clin Immunol. 1996;97:53–64.
Kinnunen T, Jutila K, Kwok WW, Rytkonen-Nissinen M, Immonen A, Saarelainen S, Narvanen A, Taivainen A, Virtanen T. Potential of an altered peptide ligand of lipocalin allergen Bos d 2 for peptide immunotherapy. J Allergy Clin Immunol. 2007;119:965–72.
Geluk A, van Meijgaarden KE, Roep BO, Ottenhoff TH. Altered peptide ligands of islet autoantigen Imogen 38 inhibit antigen specific T cell reactivity in human type-1 diabetes. J Autoimmun. 1998;11:353–61.
Kim HJ, Antel JP, Duquette P, Alleva DG, Conlon PJ, Bar-Or A. Persistence of immune responses to altered and native myelin antigens in patients with multiple sclerosis treated with altered peptide ligand. Clin Immunol. 2002;104:105–14.
Prakken BJ, Roord S, van Kooten PJ, Wagenaar JP, van Eden W, Albani S, Wauben MH. Inhibition of adjuvant-induced arthritis by interleukin-10-driven regulatory cells induced via nasal administration of a peptide analog of an arthritis-related heat-shock protein 60 T cell epitope. Arthritis Rheum. 2002;46:1937–46.
Ohnishi Y, Tsutsumi A, Matsumoto I, Goto D, Ito S, Kuwana M, Uemura Y, Nishimura Y, Sumida T. Altered peptide ligands control type II collagen-reactive T cells from rheumatoid arthritis patients. Mod Rheumatol. 2006;16:226–8.
Boots AM, Hubers H, Kouwijzer M, den Hoed-vanZandbrink L, Westrek-Esselink BM, van Doorn C, Stenger R, Bos ES, van Lierop MJ, Verheijden GF, Timmers CM, van Staveren CJ. Identification of an altered peptide ligand based on the endogenously presented, rheumatoid arthritis-associated, human cartilage glycoprotein-39(263–275) epitope: an MHC anchor variant peptide for immune modulation. Arthritis Res Ther. 2007;9:R71.
Yu Z, Maoui M, Zhao ZJ, Li Y, Shen SH. SHP-1 dephosphorylates 3BP2 and potentially downregulates 3BP2-mediated T cell antigen receptor signaling. Febs J. 2006;273:2195–205.
Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, McFarland HF, Martin R. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–75.
Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, Steinman L. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The altered peptide ligand in relapsing MS study group. Nat Med. 2000;6:1176–82.
Walter M, Philotheou A, Bonnici F, Ziegler AG, Jimenez R. No effect of the altered peptide ligand NBI-6024 on beta-cell residual function and insulin needs in new-onset type 1 diabetes. Diabetes Care. 2009;32:2036–40.
Crowe PD, Qin Y, Conlon PJ, Antel JP. NBI-5788, an altered MBP83–99 peptide, induces a T-helper 2-like immune response in multiple sclerosis patients. Ann Neurol. 2000;48:758–65.
Kim HJ, Antel JP, Duquette P, Alleva DG, Conlon PJ, Bar-Or A. Persistence of immune responses to altered and native myelin antigens in patients with multiple sclerosis treated with altered peptide ligand. Clin Immunol. 2002;104:105–14.
Pedotti R, Mitchell D, Wedemeyer J, Karpuj M, Chabas D, Hattab EM, Tsai M, Galli SJ, Steinman L. An unexpected version of horror autotoxicus: anaphylactic shock to a self-peptide. Nat Immunol. 2001;2:216–22.
Pedotti R, DeVoss JJ, Youssef S, Mitchell D, Wedemeyer J, Madanat R, Garren H, Fontoura P, Tsai M, Galli SJ, Sobel RA, Steinman L. Multiple elements of the allergic arm of the immune response modulate autoimmune demyelination. Proc Natl Acad Sci USA. 2003;100:1867–72.
Liu E, Moriyama H, Abiru N, Miao D, Yu L, Taylor RM, Finkelman FD, Eisenbarth GS. Anti-peptide autoantibodies and fatal anaphylaxis in NOD mice in response to insulin self-peptides B:9–23 and B:13–23. J Clin Invest. 2002;110:1021–7.
Margot CD, Ford ML, Evavold BD. Amelioration of established experimental autoimmune encephalomyelitis by an MHC anchor-substituted variant of proteolipid protein 139–151. J Immunol. 2005;174:3352–8.
Leech MD, Chung CY, Culshaw A, Anderton SM. Peptide-based immunotherapy of experimental autoimmune encephalomyelitis without anaphylaxis. Eur J Immunol. 2007;37:3576–81.
Ford ML, Evavold BD. Regulation of polyclonal T cell responses by an MHC anchor-substituted variant of myelin oligodendrocyte glycoprotein 35–55. J Immunol. 2003;171:1247–54.
Acknowledgments
The authors would like to thank the members of the Evavold lab for their critical reading of the manuscript. Funding was provided by the National Institutes of Health grant numbers NS 062358 and NS 071518 and the National Multiple Sclerosis Society grant numbers RG4047 and RG 4482.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Edwards, L.J., Evavold, B.D. T cell recognition of weak ligands: roles of signaling, receptor number, and affinity. Immunol Res 50, 39–48 (2011). https://doi.org/10.1007/s12026-011-8204-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-011-8204-3