Abstract
Experimental and epidemiological studies indicate a strong link between chronic inflammation and tumor progression. Human colorectal cancer (CRC), a major cause of cancer-related death in Western countries, represents a paradigm for this link. Key features of cancer-related inflammation in CRC are the activation of transcription factors (e.g. NF-κB, STAT3), the expression of inflammatory cytokines and chemokines (e.g. TNFα, IL-6, CCL2, CXCL8) as well as a prominent leukocyte infiltrate. While considerable evidence indicates that the presence of lymphocytes of adaptive immunity may positively influence patient survival and clinical outcome in CRC, the role of tumor-associated macrophages (TAM) and of other lymphoid populations (e.g. Th17, Treg) is still unclear. In this review we will summarize the different and controversial effects that TAM play in CRC-related inflammation and progression of disease. The characterization of the most relevant inflammatory pathways in CRC is instrumental for the identification of new target molecules that could lead to improved diagnosis and treatment.



Similar content being viewed by others
References
Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW (2005) Colorectal cancer. Lancet 365:153–165
Calvert PM, Frucht H (2002) The genetics of colorectal cancer. Ann Intern Med 137:603–612
Lynch HT, de la Chapelle A (2003) Hereditary colorectal cancer. N Engl J Med 348:919–932
Rustgi AK (2007) The genetics of hereditary colon cancer. Genes Dev 21:2525–2538
Soreide K, Janssen EA, Soiland H, Korner H, Baak JP (2006) Microsatellite instability in colorectal cancer. Br J Surg 93:395–406
Laghi L, Bianchi P, Malesci A (2003) Gender difference for promoter methylation pattern of hMLH1 and p16 in sporadic MSI colorectal cancer. Gastroenterology 124:1165–1166
Westra JL, Plukker JT, Buys CH, Hofstra RM (2004) Genetic alterations in locally advanced stage II/III colon cancer: a search for prognostic markers. Clin Colorectal Cancer 4:252–259
Malesci A et al. (2007) Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res 13:3831–3839
Prall F et al. (2004) Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol 35:808–816
Dolcetti R et al. (1999) High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol 154:1805–1813
Banerjea A et al. (2004) Colorectal cancers with microsatellite instability display mRNA expression signatures characteristic of increased immunogenicity. Mol Cancer 3:21
Muller A, Fishel R (2002) Mismatch repair and the hereditary non-polyposis colorectal cancer syndrome (HNPCC). Cancer Invest 20:102–109
Wang D, Dubois RN, Richmond A (2009) The role of chemokines in intestinal inflammation and cancer. Curr Opin Pharmacol 9:688–696
Meira LB et al. (2008) DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest 118:2516–2525
Talmadge JE, Donkor M, Scholar E (2007) Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev 26:373–400
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357:539–545
Balkwill F, Charles KA, Mantovani A (2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7:211–217
DeNardo DG, Johansson M, Coussens LM (2008) Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev 27:11–18
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444
Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5:749–759
Witz IP (2009) The tumor microenvironment: the making of a paradigm. Cancer Microenviron 2(Suppl 1):9–17
Chan AT, Ogino S, Fuchs CS (2007) Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med 356:2131–2142
Flossmann E, Rothwell PM (2007) Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 369:1603–1613
Sparmann A, Bar-Sagi D (2004) Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 6:447–458
Sumimoto H, Imabayashi F, Iwata T, Kawakami Y (2006) The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med 203:1651–1656
Shchors K, Shchors E, Rostker F, Lawlor ER, Brown-Swigart L, Evan GI (2006) The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1beta. Genes Dev 20:2527–2538
Borrello MG et al. (2005) Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci U S A 102:14825–14830
Germano G et al. (2010) Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res 70:2235–2244
Guerra C et al. (2007) Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11:291–302
Bierie B, Moses HL (2006) TGF-beta and cancer. Cytokine Growth Factor Rev 17:29–40
Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W (2003) Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature 425:307–311
Schioppa T et al. (2003) Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med 198:1391–1402
Gupta RB et al. (2007) Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology 133:1099–1105, quiz 1340-1
Itzkowitz SH, Harpaz N (2004) Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology 126:1634–1648
Bernstein CN, Blanchard JF, Kliewer E, Wajda A (2001) Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 91:854–862
Terzic J, Grivennikov S, Karin E, Karin M (2010) Inflammation and colon cancer. Gastroenterology 138:2101–2114.e5
Bottazzi B et al. (1983) Regulation of the macrophage content of neoplasms by chemoattractants. Science 220:210–212
Rollins BJ, Sunday ME (1991) Suppression of tumor formation in vivo by expression of the JE gene in malignant cells. Mol Cell Biol 11:3125–3131
Negus RP et al. (1995) The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest 95:2391–2396
Opdenakker G, Van Damme J (1992) Chemotactic factors, passive invasion and metastasis of cancer cells. Immunol Today 13:463–464
Mantovani A, Bonecchi R, Locati M (2006) Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol 6:907–918
McConnell BB, Yang VW (2009) The role of inflammation in the pathogenesis of colorectal cancer. Curr Colorectal Cancer Rep 5:69–74
Popivanova BK et al. (2009) Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Res 69:7884–7892
Erreni M et al. (2009) Expression of chemokines and chemokine receptors in human colon cancer. Methods Enzymol 460:105–121
Kim J et al. (2005) Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol 23:2744–2753
Schimanski CC et al. (2005) Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res 11:1743–1750
Gunther K et al. (2005) Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. Int J Cancer 116:726–733
Kawada K et al. (2007) Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene 26:4679–4688
Sturm A, Baumgart DC, d’Heureuse JH, Hotz A, Wiedenmann B, Dignass AU (2005) CXCL8 modulates human intestinal epithelial cells through a CXCR1 dependent pathway. Cytokine 29:42–48
Zipin-Roitman A et al. (2007) CXCL10 promotes invasion-related properties in human colorectal carcinoma cells. Cancer Res 67:3396–3405
Vetrano S et al. (2010) The lymphatic system controls intestinal inflammation and inflammation-associated Colon Cancer through the chemokine decoy receptor D6. Gut 59:197–206
Balkwill F (2009) Tumour necrosis factor and cancer. Nat Rev Cancer 9:361–371
Popivanova BK et al. (2008) Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 118:560–570
Greten FR et al. (2004) IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118:285–296
Pikarsky E et al. (2004) NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 431:461–466
Fukata M, Abreu MT (2008) Role of Toll-like receptors in gastrointestinal malignancies. Oncogene 27:234–243
Rakoff-Nahoum S, Medzhitov R (2007) Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science 317:124–127
Huang B et al. (2005) Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res 65:5009–5014
Thomassen E, Renshaw BR, Sims JE (1999) Identification and characterization of SIGIRR, a molecule representing a novel subtype of the IL-1R superfamily. Cytokine 11:389–399
Mantovani A, Locati M, Polentarutti N, Vecchi A, Garlanda C (2004) Extracellular and intracellular decoys in the tuning of inflammatory cytokines and Toll-like receptors: the new entry TIR8/SIGIRR. J Leukoc Biol 75:738–742
Polentarutti N et al. (2003) Unique pattern of expression and inhibition of IL-1 signaling by the IL-1 receptor family member TIR8/SIGIRR. Eur Cytokine Netw 14:211–218
Wald D et al. (2003) SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol 4:920–927
Garlanda C et al. (2004) Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci U S A 101:3522–3526
Garlanda C et al. (2007) Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res 67:6017–6021
Iglesias D, Nejda N, Azcoita MM, Schwartz SJ, Gonzalez-Aguilera JJ, Fernandez-Peralta AM (2009) Effect of COX2-765G>C and c.3618A>G polymorphisms on the risk and survival of sporadic colorectal cancer. Cancer Causes Control 20:1421–1429
Arber N et al. (2006) Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med 355:885–895
Benelli R (2007) Aspirin, COX-2, and the risk of colorectal cancer. N Engl J Med 357:824–825, author reply 824-5
Pereira C, Medeiros RM, Dinis-Ribeiro MJ (2009) Cyclooxygenase polymorphisms in gastric and colorectal carcinogenesis: are conclusive results available? Eur J Gastroenterol Hepatol 21:76–91
Cross JT, Poole EM, Ulrich CM (2008) A review of gene-drug interactions for nonsteroidal anti-inflammatory drug use in preventing colorectal neoplasia. Pharmacogenomics J 8:237–247
Hoebe K, Janssen E, Beutler B (2004) The interface between innate and adaptive immunity. Nat Immunol 5:971–974
Kishimoto T (2005) Interleukin-6: from basic science to medicine—40 years in immunology. Annu Rev Immunol 23:1–21
McLoughlin RM et al. (2003) Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J Clin Invest 112:598–607
Mantovani A, Sica A, Locati M (2005) Macrophage polarization comes of age. Immunity 23:344–346
Atreya R et al. (2000) Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med 6:583–588
Schneider MR, Hoeflich A, Fischer JR, Wolf E, Sordat B, Lahm H (2000) Interleukin-6 stimulates clonogenic growth of primary and metastatic human colon carcinoma cells. Cancer Lett 151:31–38
Hsu CP, Chung YC (2006) Influence of interleukin-6 on the invasiveness of human colorectal carcinoma. Anticancer Res 26:4607–4614
Brozek W, Bises G, Girsch T, Cross HS, Kaiser HE, Peterlik M (2005) Differentiation-dependent expression and mitogenic action of interleukin-6 in human colon carcinoma cells: relevance for tumour progression. Eur J Cancer 41:2347–2354
Knupfer H, Preiss R (2010) Serum interleukin-6 levels in colorectal cancer patients—a summary of published results. Int J Colorectal Dis 25:135–140
Naugler WE et al. (2007) Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317:121–124
Grivennikov S et al. (2009) IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15:103–113
Bollrath J et al. (2009) gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 15:91–102
Bromberg J, Wang TC (2009) Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell 15:79–80
Saraiva M, O’Garra A (2010) The regulation of IL-10 production by immune cells. Nat Rev Immunol 10:170–181
Sandel MH et al. (2005) Natural killer cells infiltrating colorectal cancer and MHC class I expression. Mol Immunol 42:541–546
Coca S et al. (1997) The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer 79:2320–2328
Fernandez-Acenero MJ, Galindo-Gallego M, Sanz J, Aljama A (2000) Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer 88:1544–1548
Nagtegaal ID et al. (2001) Local and distant recurrences in rectal cancer patients are predicted by the nonspecific immune response; specific immune response has only a systemic effect—a histopathological and immunohistochemical study. BMC Cancer 1:7
Gounaris E et al. (2007) Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci U S A 104:19977–19982
Canna K et al. (2005) The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer 92:651–654
Chiba T et al. (2004) Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer 91:1711–1717
Naito Y et al. (1998) CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 58:3491–3494
Galon J et al. (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Pages F et al. (2005) Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353:2654–2666
Laghi L et al. (2009) CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol 10:877–884
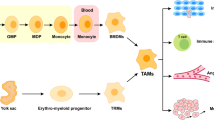
Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L (1992) The origin and function of tumor-associated macrophages. Immunol Today 13:265–270
Allavena P, Sica A, Solinas G, Porta C, Mantovani A (2008) The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol 66:1–9
Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4:71–78
Dinapoli MR, Calderon CL, Lopez DM (1996) The altered tumoricidal capacity of macrophages isolated from tumor-bearing mice is related to reduce expression of the inducible nitric oxide synthase gene. J Exp Med 183:1323–1329
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23:549–555
Sica A, Schioppa T, Mantovani A, Allavena P (2006) Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 42:717–727
Condeelis J, Pollard JW (2006) Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124:263–266
Fu YX, Cai JP, Chin YH, Watson GA, Lopez DM (1992) Regulation of leukocyte binding to endothelial tissues by tumor-derived GM-CSF. Int J Cancer 50:585–588
Yaal-Hahoshen N et al. (2006) The chemokine CCL5 as a potential prognostic factor predicting disease progression in stage II breast cancer patients. Clin Cancer Res 12:4474–4480
Scholl SM, Crocker P, Tang R, Pouillart P, Pollard JW (1993) Is colony-stimulating factor-1 a key mediator of breast cancer invasion and metastasis? Mol Carcinog 7:207–211
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3:23–35
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5:953–964
Biswas SK, Sica A, Lewis CE (2008) Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol 180:2011–2017
Goerdt S, Orfanos CE (1999) Other functions, other genes: alternative activation of antigen-presenting cells. Immunity 10:137–142
O’Sullivan C, Lewis CE (1994) Tumour-associated leucocytes: friends or foes in breast carcinoma. J Pathol 172:229–235
Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL (1996) Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 56:4625–4629
Mantovani A (1994) Tumor-associated macrophages in neoplastic progression: a paradigm for the in vivo function of chemokines. Lab Invest 71:5–16
Konur A, Kreutz M, Knuchel R, Krause SW, Andreesen R (1998) Cytokine repertoire during maturation of monocytes to macrophages within spheroids of malignant and non-malignant urothelial cell lines. Int J Cancer 78:648–653
Wyckoff JB et al. (2007) Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res 67:2649–2656
Kaplan RN et al. (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438:820–827
Kaplan RN, Rafii S, Lyden D (2006) Preparing the “soil”: the premetastatic niche. Cancer Res 66:11089–11093
Mantovani A, Schioppa T, Porta C, Allavena P, Sica A (2006) Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev 25:315–322
Bronte V, Zanovello P (2005) Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol 5:641–654
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174
Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S (2007) Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol 179:977–983
Gounaris E et al. (2008) Live imaging of cysteine-cathepsin activity reveals dynamics of focal inflammation, angiogenesis, and polyp growth. PLoS One 3:e2916
Balkwill F (2004) Cancer and the chemokine network. Nat Rev Cancer 4:540–550
Steidl C et al. (2010) Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 362:875–885
Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R (2007) High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res 13:1472–1479
Ohno S et al. (2003) The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer Res 23:5015–5022
Barbera-Guillem E, Nyhus JK, Wolford CC, Friece CR, Sampsel JW (2002) Vascular endothelial growth factor secretion by tumor-infiltrating macrophages essentially supports tumor angiogenesis, and IgG immune complexes potentiate the process. Cancer Res 62:7042–7049
Jedinak A, Dudhgaonkar S, Sliva D (2010) Activated macrophages induce metastatic behavior of colon cancer cells. Immunobiology 215:242–249
Xu L et al. (2009) Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1alpha, CXCR4, CXCL6, and neuropilin 1 in tumors from patients with rectal cancer. Cancer Res 69:7905–7910
Popovic ZV et al. (2007) Sulfated glycosphingolipid as mediator of phagocytosis: SM4s enhances apoptotic cell clearance and modulates macrophage activity. J Immunol 179:6770–6782
Herbeuval JP, Lelievre E, Lambert C, Dy M, Genin C (2004) Recruitment of STAT3 for production of IL-10 by colon carcinoma cells induced by macrophage-derived IL-6. J Immunol 172:4630–4636
Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7:415–428
Massague J (2008) TGFbeta in cancer. Cell 134:215–230
Reinacher-Schick A et al. (2004) Loss of Smad4 correlates with loss of the invasion suppressor E-cadherin in advanced colorectal carcinomas. J Pathol 202:412–420
Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A (2006) Transforming growth factor-beta employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol 174:175–183
Illemann M et al. (2006) MMP-9 is differentially expressed in primary human colorectal adenocarcinomas and their metastases. Mol Cancer Res 4:293–302
Kaler P, Godasi BN, Augenlicht L, Klampfer L (2009) The NF-kappaB/AKT-dependent induction of Wnt signaling in colon cancer cells by macrophages and IL-1beta. Cancer Microenviron
Bienz M, Clevers H (2000) Linking colorectal cancer to Wnt signaling. Cell 103:311–320
Segditsas S, Tomlinson I (2006) Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene 25:7531–7537
Pancione M et al. (2009) Reduced beta-catenin and peroxisome proliferator-activated receptor-gamma expression levels are associated with colorectal cancer metastatic progression: correlation with tumor-associated macrophages, cyclooxygenase 2, and patient outcome. Hum Pathol 40:714–725
Ohtani H, Naito Y, Saito K, Nagura H (1997) Expression of costimulatory molecules B7-1 and B7-2 by macrophages along invasive margin of colon cancer: a possible antitumor immunity? Lab Invest 77:231–241
Sugita J et al. (2002) Close association between Fas ligand (FasL; CD95L)-positive tumor-associated macrophages and apoptotic cancer cells along invasive margin of colorectal carcinoma: a proposal on tumor-host interactions. Jpn J Cancer Res 93:320–328
Khorana AA, Ryan CK, Cox C, Eberly S, Sahasrabudhe DM (2003) Vascular endothelial growth factor, CD68, and epidermal growth factor receptor expression and survival in patients with Stage II and Stage III colon carcinoma: a role for the host response in prognosis. Cancer 97:960–968
Funada Y, Noguchi T, Kikuchi R, Takeno S, Uchida Y, Gabbert HE (2003) Prognostic significance of CD8+ T cell and macrophage peritumoral infiltration in colorectal cancer. Oncol Rep 10:309–313
Zhou Q et al. (2010) The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Transl Med 8:13
Bailey C et al. (2007) Chemokine expression is associated with the accumulation of tumour associated macrophages (TAMs) and progression in human colorectal cancer. Clin Exp Metastasis 24:121–130
Mantovani A, Sica A, Allavena P, Garlanda C, Locati M (2009) Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol 70:325–330
Dunn GP, Old LJ, Schreiber RD (2004) The three Es of cancer immunoediting. Annu Rev Immunol 22:329–360
Gabrilovich DI et al. (2007) The terminology issue for myeloid-derived suppressor cells. Cancer Res 67:425, author reply 426
Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F (2007) Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol 8:942–949
Manel N, Unutmaz D, Littman DR (2008) The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol 9:641–649
Santarlasci V et al. (2009) TGF-beta indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur J Immunol 39:207–215
Das J et al. (2009) Transforming growth factor beta is dispensable for the molecular orchestration of Th17 cell differentiation. J Exp Med 206:2407–2416
Langrish CL et al. (2005) IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201:233–240
Buonocore S et al. (2010) Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464:1371–1375
Wang YQ et al. (2003) Induction of systemic immunity by expression of interleukin-23 in murine colon carcinoma cells. Int J Cancer 105:820–824
Langowski JL et al. (2006) IL-23 promotes tumour incidence and growth. Nature 442:461–465
Stanilov N, Miteva L, Mintchev N, Stanilova S (2009) High expression of Foxp3, IL-23p19 and survivin mRNA in colorectal carcinoma. Int J Colorectal Dis 24:151–157
Colonna M (2009) Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity 31:15–23
Kryczek I, Wei S, Szeliga W, Vatan L, Zou W (2009) Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood 114:357–359
Lee JW et al. (2008) Differential regulation of chemokines by IL-17 in colonic epithelial cells. J Immunol 181:6536–6545
Bettelli E et al. (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238
Veldhoen M, Stockinger B (2006) TGFbeta1, a “Jack of all trades”: the link with pro-inflammatory IL-17-producing T cells. Trends Immunol 27:358–361
Savage ND et al. (2008) Human anti-inflammatory macrophages induce Foxp3+ GITR+CD25+ regulatory T cells, which suppress via membrane-bound TGFbeta-1. J Immunol 181:2220–2226
Izcue A, Coombes JL, Powrie F (2009) Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol 27:313–338
Loddenkemper C, Schernus M, Noutsias M, Stein H, Thiel E, Nagorsen D (2006) In situ analysis of FOXP3+ regulatory T cells in human colorectal cancer. J Transl Med 4:52
Salama P et al. (2009) Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 27:186–192
Erdman SE et al. (2005) CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res 65:3998–4004
Chaput N et al. (2009) Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut 58:520–529
Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC (2010) Regulatory T cells in cancer. Adv Cancer Res 107:57–117
Acknowledgments
This work was supported by Associazione Italiana Ricerca Cancro (AIRC) Italy to PA and AM; grants from the European Community FP6 Project ATTACK-018914; Ministry of Health and Istituto Superiore Sanità Italy (Project oncology 2006 and Alleanza Contro il Cancro).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erreni, M., Mantovani, A. & Allavena, P. Tumor-associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenvironment 4, 141–154 (2011). https://doi.org/10.1007/s12307-010-0052-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-010-0052-5