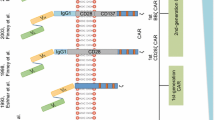
Abstract
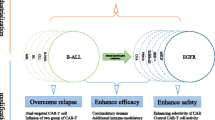
Adoptive immunotherapy with chimeric antigen receptor-modified (CAR)-T cells is a rapidly growing therapeutic approach to treating patients with refractory cancer, with over 100 clinical trials in various malignancies in progress. The enthusiasm for CAR-T cells has been driven by the clinical success of CD19-targeted CAR-T cell therapy in B-cell acute lymphoblastic leukemia, and the promising data in B-cell non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Despite the success of targeting CD19 with CAR-T cells in early clinical studies, many challenges remain to improve outcomes, reduce toxicity, and determine the appropriate settings for CAR-T cell immunotherapy. Reviewing the lessons learned thus far in CD19 CAR-T cell trials and how some of these challenges may be overcome will help guide the development of CAR-T cell therapy for malignancies of B-cell origin, as well as for other hematopoietic and non-hematopoietic cancers.


Similar content being viewed by others
References
Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringdén O, Rozman C, Speck B. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62.
Turtle CJ. Chimeric antigen receptor modified T cell therapy for B cell malignancies. Int J Hematol. 2014;99:132–40.
Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–71.
Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–6.
Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR, Forman SJ. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transpl. 2010;16:1245–56.
Grupp SA, Kalos M, Barrett DM, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18.
Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17.
Porter DL, Hwang W-T, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–28.
Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20.
Turtle CJ, Hanafi L-A, Berger C, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8:355ra116.
Turtle CJ, Hanafi L-A, Berger C, et al. CD19 CAR-T cells of defined CD4 + :CD8 + composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–38.
Till BG, Jensen MC, Wang J, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940–50.
Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15:1145–54.
Stamenkovic I, Seed B. CD19, the earliest differentiation antigen of the B cell lineage, bears three extracellular immunoglobulin-like domains and an Epstein-Barr virus-related cytoplasmic tail. J Exp Med. 1988;168:1205–10.
Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28.
Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–95.
Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:22425.
Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–79.
Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38.
Park JH, Riviere I, Wang X, Purdon T, Sadelain M, Brentjens RJ. Impact of disease burden on long-term outcome of 19-28z CAR modified T cells in adult patients with relapsed B-ALL. J Clin Oncol. 2016;34:7003 (suppl–abstr).
Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127:3312–20.
Geyer MB, Brentjens RJ. Review: Current clinical applications of chimeric antigen receptor (CAR) modified T cells. Cytotherapy. 2016;18:1393–409.
Lee DW, Stetler-Stevenson M, Yuan CM, et al. Safety and response of incorporating CD19 chimeric antigen receptor T cell therapy in typical salvage regimens for children and young adults with acute lymphoblastic leukemia. Blood. 2015;126:684.
Brudno JN, Somerville R, Shi V, et al. Allogeneic T-cells expressing an anti-CD19 chimeric antigen receptor cause remissions of B-Cell malignancies after allogeneic hematopoietic stem cell transplantation without causing graft-versus-host disease. Blood. 2015;126:99.
Brudno JN, Somerville RPT, Shi V, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. Clin Oncol. 2016;34:1112–21.
Maude SL, Teachey DT, Rheingold SR, et al. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. J Clin Oncol. 2016;34:3011 (suppl–abstr).
Frey NV, Shaw PA, Hexner EO, et al. Optimizing chimeric antigen receptor (CAR) T cell therapy for adult patients with relapsed or refractory (r/r) acute lymphoblastic leukemia (ALL). J Clin Oncol. 2016;34:7002 (suppl–abstr).
Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, Riddell SR. Chimeric antigen receptor-modified T cells derived from defined CD8 + and CD4 + subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500.
Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–9.
Kochenderfer J, Somerville R, Lu T, et al. Anti-CD19 chimeric antigen receptor T cells preceded by low-dose chemotherapy to induce remissions of advanced lymphoma. J Clin Oncol. 2016;34:LBA3010 (suppl–abstr).
Schuster SJ, Svoboda J, Dwivedy Nasta S, et al. Sustained remissions following chimeric antigen receptor modified t cells directed against CD19 (CTL019) in patients with relapsed or refractory CD19+ lymphomas. Blood. 2015;126:183.
Porter DL, Frey NV, Melenhorst JJ, et al. Randomized, phase II dose optimization study of chimeric antigen receptor (CAR) modified T cells directed against CD19 in patients (pts) with relapsed, refractory (R/R) CLL. J Clin Oncol. 2016;34:3009 (suppl–abstr).
Geyer MB, Park JH, Riviere I, Wang X, Purdon T, Sadelain M, Brentjens RJ. Updated results: phase I trial of autologous CD19-targeted CAR T cells in patients with residual CLL following initial purine analog-based therapy. J Clin Oncol. 2016;34:7526 (suppl–abstr).
Turtle CJ, Hanafi L-A, Berger C, et al. Rate of durable complete response in ALL, NHL, and CLL after immunotherapy with optimized lymphodepletion and defined composition of CD19 CAR-T cells. J Clin Oncol. 2016;34:102 (suppl–abstr).
Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–7.
Cieri N, Camisa B, Cocchiarella F, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121:573–84.
Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–33.
Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–44.
Gargett T, Yu W, Dotti G, Yvon ES, Christo SN, Hayball JD, Lewis ID, Brenner MK, Brown MP. GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol Ther. 2016;24:1135–49.
Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–95.
Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–10.
Haso W, Lee DW, Shah NN, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–74.
Zah E, Lin M-Y, Silva-Benedict A, Jensen MC, Chen YY. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res. 2016;4:498–508.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
KAH is supported by the University of British Columbia, Clinical Investigator Program Fellowship.
Conflict of interest
KAH declares no conflict of interest. CJT receives research funding from Juno Therapeutics and has received payment for participation on advisory boards and for speaking at educational events. CJT has patents pending related to CAR-T cells.
Rights and permissions
About this article
Cite this article
Hay, K.A., Turtle, C.J. Chimeric Antigen Receptor (CAR) T Cells: Lessons Learned from Targeting of CD19 in B-Cell Malignancies. Drugs 77, 237–245 (2017). https://doi.org/10.1007/s40265-017-0690-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0690-8