Abstract
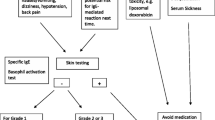
With the FDA approval of Rituximab in 1998 for the treatment of lymphoma, and Trastuzumab in 1999 for the treatment of breast cancer, monoclonal antibodies were officially added to the therapeutic armamentarium against malignancy. Most of the side effects associated with these agents are due to antigen-antibody interactions on specific cells and tissues. One of the most predictable side effects of these products is a constellation of various systemic effects including flu-like syumptoms such as headache, fever, sweats, skin rash, shortness of breath, hypotension, nausea, and asthenia that occurs with the first infusion of such products. Rarely severe hypotension, bronchospasm, and hypoxia and even death have occurred. The pathophysiology of these reactions appears to be secondary to the release of cytokines as the antibodies bind do circulating antigen-expressing cells that are then removed in the reticuloendothelial system of the lungs, spleen and liver. In patients with large numbers of antigen-dense cells that have a high mitotic index, such as prolymphocytic leukemia, mantle cell lymphoma, or lymphosarcoma cell leukemia, there is a risk of true tumor lysis syndrome. One should be particularly cautious when treating patients with high numbers of circulating antigen-expressing cells in the setting of underlying cardiovascular or respiratory disease.
Similar content being viewed by others
References
Dillman RO: Monoclonal antibodies for treating cancer. Ann Intern Med 111: 592–603, 1989
Dillman RO: Antibodies as cytotoxic therapy. J Clin Oncol 12: 1497–1515, 1994
Dillman RO: Whither magic bullets and guided missiles: monoclonal antibodies 20 years later. Cancer Biother 10: 177–180, 1995
Dillman RO: Antibody therapy. In: Oldham RK (ed) Principles of Cancer Biotherapy, 3rd edn, Kluwer Academic Publishers, The Netherlands, 1998, pp 284–317
Meredith RF, LoBuglio AF: Recent progress in radioimmunotherapy for cancer. Oncology 11: 979–987, 1997
Wilder RB, DeNardo GL, DeNardo SJ: Radioimmunotherapy: recent results and future directions. J Clin Oncol 14: 1383–1400, 1996
Spitler LE: Immunotoxins. In: Oldham RK (ed) Principles of Cancer Biotherapy, 3rd edn, Kluwer Academic Publishers, The Netherlands, 1998, pp 318–337
Frankel AE: Immunotoxin therapy of cancer. Oncology 7: 69–78, 1993
Oldham RK, Foon K: Drug immunoconjugates. In: Oldham RK (ed) Principles of Cancer Biotherapy, 3rd edn, Kluwer Academic Publishers, The Netherlands, 1998, pp 338–347
Dillman RO: Unconjugated monoclonal antibodies for the treatment of hematologic and solid malignancies. Am Soc Clin Oncol 1999 Education Book, 1999, pp 461–468
Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg, Grillo-Lopez A, Levy R: Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent lymphoma. Blood 84: 2457–2466, 1994
Maloney DG, Griullo-Lopez AJ, Bodkin DJ, White CA, Liles T-M, Royston I, Varns C, Rosenberg J, Levy R: IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J Clin Oncol 15: 3266–3274, 1997
Dillman RO: Magic bullets at last! Finally — approval of a monoclonal antibody for the treatment of cancer!!! Cancer Biother Radiopharm 12: 223–225, 1997
McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Breckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK: Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 16: 2825–2833, 1988
Coiffier B, Haioun C, Ketterer N, Engert A, Tillly H, Ma D, Johnson P, Lister A, Feuring-Buske M, Radford JA, Capdeville R, Diehl V, Reyes F: Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood 92: 1927–1932, 1998
Piro L, White CA, Grillo-Lopez AJ, Janakiraman N, Beck TM, Saven A, Shuey S, Czuczman M, Lynch JW, Kolitz JE, Jain V: Rituximab in patients with relapsed low-grade or follicular non-Hodgkin's lymphoma: response rate and duration with a weekly times 8 dosing regimen. Proc Am Soc Clin Oncol 18: 14a, 1999
Davis TA, White CA, Grillo-Lopex AJ, Velasquez WS, Link B, Maloney DG, Dillman RO, Williams ME, Mohrbacher A, Weaver R, Dowden S, Levy R: Single-agent monoclonal antibody efficacy in bulky non-Hodgkin's lymphoma: results of a phase II trial of Rituximab. J Clin Oncol 1851–1857, 1999
Davis T, Levy R, White CA, Czuczman M, McLaughlin P, Link B, Varns C, Weaver R, Grillo-Lopez AJ: Rituximab: phase II retreatment study in patients with low-grade or follicular NHL. Blood 92(10, suppl 1): 414a, 1998
Czuczman M, Grillo-Lopez AJ, White CA, Saleh M, Gordon L, LoBuglio AF, Jonas C, Dallaire B, Varns C: Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol 17: 268–276, 1999
Link B, Grossbard ML, Fisher RI, Czuczman M, Gilman P, Lowed AM, Vose JM: Phase II pilot study of the safety and efficacy of Rituximab in combination with CHOP chemotherapy in patients with previously untreated intermediate-or high-grade NHL. Proc Am Soc Clin Oncol 17: 3a, 1998
Byrd JC, White CA, Link B, Thomas S, Valasquez WS, Rosenberg, Grillo-Lopez AJ: Rituximab therapy in previously treated Waldenstrom's macroglobulinemia: preliminary evidence of activity. Blood 92(10, suppl 1): 106a, 1998
O'Brien S, Freireich E, Andreeff M, Lerner S, Keating M: Phase I/II study of Rituxan in chronic lymphocytic leukemia. Blood 92(10, suppl 1): 105a, 1998
Dillman RO: Perceptions of Herceptin® a monoclonal antibody for the treatment of breast cancer. Cancer Biother 14: 5–10, 1999
Coussens L, Yang-Feng Tl, Liao YC, Chen F, Ray A, McGrath J, Seeburg PH, Libermann TA, Schlessinger J, Francke U, et al.: Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science 230: 1132–1139, 1985
Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, Rosen PP, Twaddell T, Henderson IC, Norton L: Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu overexpressing metastatic breast cancer. J Clin Oncol 14: 737–744, 1996
Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Bauthman SA, Twaddell T, Glaspy JA, Slamon DJ: Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus Cisplatin in patients with HER2/neu overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol 16: 2659–2671, 1998
Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher, Wolter JM, Paton V, Shak S, Lieberman G, Salmon DJ: Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER-2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17: 2639–2648, 1999
Norton L, Slamon D, Leyland-Jones B, Wolter J, Fleming T, Eirmann W, Baselga J, Mendelsohn J, Bajamonde A, Ash M, Shak S: Overall survival advantage to simultaneous chemotherapy plus the humanized anti-HER2 monoclonal antibody Herceptin in Her2-overexpressing metastatic breast cancer. J Clin Oncol 18: 127a, 1999
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udov J, Ullrich A, et al.: Studies of the HER2/neu proto-oncogene in human breast and ovarian cancer. Science 244: 707–712, 1989
Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ: Comparison of fluorescence in situ hybridization and immunohistochemistry for the evaluation of HER-2/neu in breast cancer. J Clin Oncol 17: 1974–1982, 1999
Woods TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ: Specificity of HercepTest in determining HER-2/neu status of breast cancers using the United States Food and Drug Administration-approved scoring system. J Clin Oncol 17: 1983–1987, 1999
Rituxan™ (Rituximab). Physician's Desk Reference 1384–1386, 1999
Herceptin® (Trastuzumab). Product Information, 1998
Dillman RO, Dillman JB, Halpern SE, Markman M, Clutter M: Toxicities and side effects associated with intravenous infusions of murine monoclonal antibodies. J Biol Response Mod 5: 73–84, 1986
Dillman RO, Beauregard JC, Jamieson M, Amox D, Halpern SE: Toxicities associated with monoclonal antibody infusions in cancer patients. Molec Biother 1: 81–85, 1988
Dillman JB: Toxicity of monoclonal antibodies in the treatment of cancer. Seminar Oncol Nurs 4: 107–111, 1988
Borden EC, Crawford J, Cross A, Dillman RO, Ernstoff M, Hawkins M, Heyman M: Toxicity of biologic response modifiers. In: Perry M (ed) Chemotherapy Handbook. Williams and Wilkins, Baltimore, pp 833–841, 1996
Dillman RO, Beauregard JC, Sobol RE, Royston I, Bartholomew RM, Hagan PS, Halpern SE: Lack of radioimmunodetection and complications associated with monoclonal anti-CEA antibody cross-reactivity with an antigen on circulating cells. Cancer Res 44: 2213–2217, 1984
Ritz J, Pesando JM, Sallan SE, Clawell LA, Notis-McConarty J, Rosenthal P, Schlossman SF: Serotherapy of acute lymphoblastic leukemia with monoclonal antibody. Blood 58: 141–152, 1981
Miller RA, Maloney DG, McKillop J, Levy R: In vivo effects of murine hybridoma monoclonal antibody in a patient with T-cell leukemia. Blood 58: 76–86, 1981
Dillman RO, Shawler DL, Sobol RE, Collins HA, Beauregard JC, Wormsley SB, Royston I: Murine monoclonal antibody therapy in two patients with chronic lymphocytic leukemia. Blood 59: 1036–1045, 1982
Miller RA, Oseroff AR, Stratte PT, Levy R: Monoclonal antibody therapeutic trials in seven patients with T-cell lymphoma. Blood 62: 988–995, 1983
Ball ED, Bernier GM, Cornwell III GG, McIntyre OR, O'Donnell JF, Fanger MW: Monoclonal antibodies to myeloid differentiation antigens: in vivo studies of three patients with acute myelogenous leukemia. Blood 62: 1203–1210, 1983
Dillman RO, Shawler DL, Dillman JB, Royston I: Therapy of chronic lymphocytic leukemia and cutaneous T-cell lymphoma with T101 monoclonal antibody. J Clin Oncol 2: 881–891, 1984
Foon KA, Schroff RW, Bunn PA, Mayer D, Abrams PB, Fer M, Ochs J, Bottino GC, Sherwin SA, Carlo DJ, Herberman RB, Oldham RK: Effects of monoclonal antibody therapy in patients with chronic lymphocytic leukemia. Blood 64: 1085–1093, 1984
Meeker TC, Lowder J, Maloney DG, Miller R, Thielemans K, Warnke R, Levy R: A clinical trial of anti-idiotype therapy for B cell malignancy. Blood 65: 1349–1363, 1985
Dillman RO, Beauregard J, Shawler DL, Halpern SE, Markman M, Ryan KP, Baird SM, Clutter M: Continuous infusion of T101 monoclonal antibody in chronic lymphocytic leukemia and cutaneous T cell lymphoma. J Biol Response Mod 5: 394–410, 1986
Waldmann TA, Goldman CK, Bongiovanni KF, Sharrow SO, Davey MP, Cease KB, Greenberg SJ, Longo DL: Therapy of patients with human T-cell lymphotropic virus I-induced adult T-cell leukemia with antitac, a monoclonal antibody to the receptor interleukin-2. Blood 72: 1805–1816, 1988
Scheinberg DA, Lovett D, Divgi CR, Graham MC, Berman E, Pentlow K, Feirt N, Finn RD, Clarkson BD, Gee TS, Larson SM, Oettgen H, Old LJ: A phase I trial of monoclonal antibody M195 in acute myelogenous leukemia: specific bone marrow targeting and internalization of radionuclide. J Clin Oncol 9: 478–490, 1991
Knox SL, Levy R, Hodgkinson S, Bell R, Brown S, Wood GS, Hoppe R, Abel FA, Steinman L, Berger RG, Gasier C, Young G, Bindl J, Hanham A, Reichert T: Observations on the effect of chimeric anti-CD4 monoclonal antibody in patients with mycosis fungoides. Blood 77: 20–30, 1991
Osterborg A, Dyer MJS, Bunjes D, Pangalis GA, Bastion Y, Catovsky D, Mellstedt H: Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia. J Clin Oncol 15: 1567–1574, 1997
Oldham RK, Foon KA, Morgan C, Woodhouse CS, Schroff RW, Abrams PG, Fer M, Schoenberger CS, Farrell M, Kimball E, Sherwin SA: Monoclonal antibody therapy of malignant melanoma: in vivo localization in cutaneous metastases after intravenous administration. J Clin Oncol 2: 1235–1244, 1984
Sears HF, Herlyn D, Steplewski Z, Koprowski H: Effects of monoclonal antibody immunotherapy on patients with gastrointestinal adenocarcinoma. J Biol Response Modif 3: 136–150, 1984
Houghton AN, Mintzer D, Cordon-Cardo C, Welt S, Fliegel B, Vadhan S, Carswell E, Melamed MR, Oettgen HF, Old LJ: Mouse monoclonal IgG3 antibody detecting GD3 ganglioside: a phase I trial in patients with malignant melanoma. Proc Natl Acad Sci USA 82: 1242–1246, 1985
Goodman GE, Beaumier P, Hellstrom I, Fernyhough B, Hellstrom K-E: Pilot trial of murine monoclonal antibodies inpatients with advanced melanoma. J Clin Oncol 3: 340–352, 1985
Cheung N-KV, Lazarus H, Miraldi FD, Abramowsky CR, Kallick S, Saarinen UM, Spitzer T, Strandjord SE, Coccia PF, Berger NA: Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. J Clin Oncol 5: 1430–1440, 1987
LoBuglio AF, Saleh MN, Lee J, Khazaeli MB, Carrano R, Holden H, Wheeler RH: Phase I trial of multiple large doses of murine monoclonal antibody CO17–1A. I. Clinical aspects. J Natl Cancer Inst 17: 932–936, 1988
Goodman GE, Hellstrom I, Brodzinsky L, Nicaise C, Kulander B, Hummel D, Hellstrom KK: Phase I trial of murine monoclonal antibody L6 in breast, colon, ovarian, and lung cancer. J Clin Oncol 8: 1083–1092, 1990
Elias DM, Hirschowitz L, Kline LE, Kroener JF, Dillman RO, Walker LE, Robb JA, Timms RM: Phase I clinical comparative study of monoclonal antibody KS1/4 and KS1/4-methotrexate immunoconjugate in patients with non-small cell lung carcinoma. Cancer Res 50: 4154–4159, 1990
Mellstedt H, Fordin J-E, Masucci G, Ragnhammar P, Fagerberg J, Hjelm A-L, Shetye J, Wersall P, Osterborg A: The therapeutic use of monoclonal antibodies in colorectal carcinoma. Semin Oncol 18: 462–477, 1991
Saleh MN, Khazaeli MB, Wheeler RH, Dropcho F, Liu T, Urist M, Miller DM, Lawson S, Dixon P, Russell CH, LoBuglio AF: Phase I trial of the murine monoclonal anti-GD2 antibody 14G2a in metastatic melanoma. Cancer Res 52: 4342–4347, 1992
Riethmuller G, Schnelder-Gadicje E, Schlimok G, Schmiegel W, Raab R, Hoffken K, Gruber R, Pichlmaier H, Hirche H, Pichlmayr R, Buggisch P, Witte J: Randomized trial of monoclonal antibody for adjuvant therapy of resected Dukes C colorectal carcinoma. Lancet 343: 1117–1183, 1994
Murray JL, Cunningham JE, Brewer H, Mujoo K, Zukiwski AA, Podoloff DA, Kasi LP, Bhadkamkar V, Fritsche HA, Bejnamin RS, Legha SS, Ater JL, Jaffe N, Itoh K, Ross MI, Bucana CD, Thompson L, Cheung L, Rosenblum MG: Phase I trial of murine monoclonal antibody 14G2a administered by prolonged intravenous infusion in patients with neuroectodermal tumor. J Clin Oncol 12: 184–193, 1994
Yu AL, Martina M, Uttenreuther-Fischer MM, Huang CS, Tsui CC, Gillies SD, Reisfeld RA, Kung FH: Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol 16: 2169–2180, 1998
Riethmuller G, Holz E, Schlimok G, Raab R, Hoffken K, Gruber R. Funke I, Pichlmaier H, Hirche H, Buggisch P, Witte J, Pichlmayr R: Monoclonal antibody therapy for resected Dukes' C colorectal cancer: seven-year outcome of a multicenter randomized trial. J Clin Oncol 16: 1788–1794, 1998
Winkler U, Jensen M, Manzke O, Tesch H, Bohlen H, Diehl V, Engert A, et al.: Severe side effects in patients with B-cell chronic lymphocytic leukemia and lymphocytosis treated with the monoclonal anti-CD20 antibody Rituximab. Blood 92(10, suppl 1): 285b, 1998
Byrd JC, Waselenko JK, Maneatis TA, Murphy T, Weickum R, Ward FT, White CA: Rituximab therapy in hematologic malignancy patients with circulating blood tumor cells: association with increased infusion-related side effects and rapid tumor lysis. Blood 92(10, suppl 1): 106a, 1998
Lee KS: Requirement for neuroregulin receptor, erbB2, in neural and cardiac development. Nature 379: 394–396, 1995
Dillman RO, Shawler DL, Johnson DE, Meyer DL, Koziol JA, Frincke JE: Preclinical trials with combinations and conjugates of T101 and doxorubicin. Cancer Res 46: 4886–4891, 1986
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dillman, R.O. Infusion Reactions Associated with the Therapeutic Use of Monoclonal Antibodies in the Treatment of Malignancy. Cancer Metastasis Rev 18, 465–471 (1999). https://doi.org/10.1023/A:1006341717398
Issue Date:
DOI: https://doi.org/10.1023/A:1006341717398