Abstract
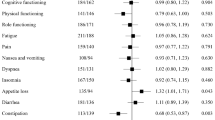
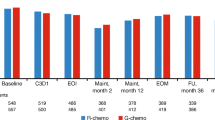
Purpose: This paper reports on the development and validation of two biologic response modifier (BRM) subscales for use with the Functional Assessment of Cancer Therapy-General (FACT-G) quality of life (QOL) questionnaire. Methods: Using the FACT-G as a base, 17 additional questions related to symptoms common to interferon and retinoid therapy were developed. Data collected at baseline (n = 191) and week 2 (n = 168) in a randomized trial of interferon ±13-cis-retinoic acid in advanced renal cell carcinoma patients were used to validate this measure. Results: Using a combined empirical and conceptual approach, the 17 questions were reduced to 13 questions consisting of two subscales: ‘BRM-physical’ (7 items; baseline coefficient alpha(α) = 0.70; week-2 α = 0.75) and ‘BRM-mental’ (6 items; baseline α = 0.79; week-2 α = 0.78). Internal consistency of the trial outcome index (TOI) combining physical well-being, functional well-being and the BRM subscales, was 0.91 for baseline assessments and 0.92 for week 2. Discriminant validity was demonstrated for the TOI by its ability to differentiate among prognostic risk groups, and for the total FACT-G, TOI and total FACT–BRM scores by their ability to distinguish between groups differing in performance, response and toxicity status. Conclusions: The ‘BRM-physical’ and ‘BRM-mental’ subscales can be combined with the FACT-G to form the ‘FACT–BRM’ scale, useful for measuring QOL in cancer patients who are receiving treatment with biologic response modifiers.
Similar content being viewed by others
References
Fairclough DL. Quality of life, cancer investigation, and clinical practice. Cancer Invest 1998; 16: 478-484.
Cella DF. The Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System. Center on Outcomes, Research, and Education (CORE), Evanston Northwestern Healthcare and Northwestern University, Version 4, 1997.
Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy Scale: Development and validation of the general measure. J Clin Oncol 1993; 11: 570-579.
Jonasch E, Haluska FG. Interferon in oncological practice: Review of interferon biology, clinical applications, and toxicities. The Oncologist 2001; 6: 34-55.
Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol 2000; 163: 408-417.
Chapman PB, Parkinson DR, Kirkwood JM. Biologic Therapy. In: Balch CM, Houghton AN, Soong S-J (eds), Cutaneous Melanoma, Philadelphia: J.B. Lippincott, 1998; 419-436.
Borden EC, Parkinson D. A perspective on the clinical effectiveness and tolerance of interferon-α. Sem Oncol 1998; 25: 3-8.
Valentine AD, Meyers CA, Kling MA, et al. Mood and cognitive side effects of interferon-α therapy. Sem Oncol 1998; 25: 39-47.
Trask P, Esper P, Riba M, Redman B. Psychiatric side effects of interferon therapy: Prevalence, proposed mechanisms, and future directions. J Clin Oncol 2000; 18: 2316-2326.
Motzer RJ, Murphy BA, Bacik J, et al. Phase III trial of interferon alfa-2a with or without 13-cis-retinoic acid for patients with advanced renal cell carcinoma. J Clin Oncol 2000; 18: 2972-2980.
Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981; 47: 207-214.
Fairclough DL, Cella DF. Functional assessment of cancer therapy (FACT-G): Non-response to individual questions. Qual Life Res 1996; 5: 321-329.
Hatcher L. A Step-by-Step Approach to Using the SAS System for Factor Analysis and Structural Equation Modeling. Cary, NC: SAS Institute Inc., 1994; 588 pp.
Cattell RB. The meaning and strategic use of factor analysis. In: Cattell RB (ed.), Handbook of Multivariate Experimental Psychology, Chicago, Rand-McNally, 1966.
Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika 1951; 16: 297-334.
Hollander M, Wolfe DA. Nonparametric Statistical Methods. New York: John Wiley and Sons, Inc., 1973; 67-74.
Johnson RA, Wichern DW. Applied Multivariate Statistical Analysis. Upper Saddle River, New Jersey: Prentice-Hall, Inc., 1992; 219-284.
Schlucter MD. Methods for the analysis of informatively censored longitudinal data. Statist Med 1992; 11: 1861-1870.
Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic significance of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999; 17: 2530-2540.
Cattell RB. The screen test for the number of factors. Multivar Behav Res 1966; 1: 245-276.
Slevin ML, Plant H, Lynch D, et al. Who should measure quality of life, the doctor or the patient? Br J Cancer 1988; 57: 109-112.
Litwin MS, Lubeck DP, Henning JM, et al. Differences in urologist and patient assessments of health related quality of life in men with prostate cancer: Results of the CaP-SURE database. J Urol 1998; 159: 1988-1992.
Bonomi AE, Cella DF, Hahn EA, et al. Multilingual translation of the functional assessment of cancer therapy (FACT) quality of life measurement system. Qual Life Res 1996; 5(3): 1-12.
Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the functional assessment of cancer therapy (FACT) measurement system. J Pain Symptom Manage 1997; 13: 63-74.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bacik, J., Mazumdar, M., Murphy, B. et al. The functional assessment of cancer therapy–BRM (FACT–BRM): A new tool for the assessment of quality of life in patients treated with biologic response modifiers. Qual Life Res 13, 137–154 (2004). https://doi.org/10.1023/B:QURE.0000015297.91158.01
Issue Date:
DOI: https://doi.org/10.1023/B:QURE.0000015297.91158.01