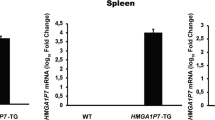
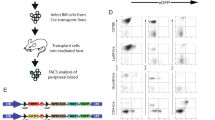
Abstract
Cancer is thought to arise from multiple genetic events that establish irreversible malignancy. A different mechanism might be present in certain leukaemias initiated by a chromosomal translocation. We have taken a new approach to determine if ablation of the genetic abnormality is sufficient for reversion by generating a conditional transgenic model of BCR–ABL1 (also known as BCR–ABL)-induced leukaemia. This oncogene1 is the result of a reciprocal translocation and is associated with different forms of leukaemia2. The most common form, p210 BCR–ABL1, is found in more than 90% of patients with chronic myelogenous leukaemia3,4 (CML) and in up to 15% of adult patients with de novoacute lymphoblastic leukaemia5 (ALL). Efforts to establish a useful transgenic model have been hampered by embryonic lethality when the oncogene is expressed during embryogenesis6,7, by reduced penetrance or by extremely long latency periods8,9. One model uses the ‘knock-in’ approach to induce leukaemia by p190 BCR–ABL1(ref. 10). Given the limitations of models with p210, we used a different experimental approach11. Lethal leukaemia developed within an acceptable time frame in all animals, and complete remission was achieved by suppression of BCR–ABL1expression, even after multiple rounds of induction and reversion. Our results demonstrate that BCR–ABL1is required for both induction and maintenance of leukaemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nowell, P.C. & Hungerford, D.A. A minute chromosome in human chronic granulocytic leukemia. Science 132, 1197–1200 (1960).
Melo, J. V. The molecular biology of chronic myeloid leukaemia. Leukemia 10, 751–756 (1996).
Shtivelman, E., Lifshitz, B., Gale, R.P. & Canaani, E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature 315, 550–554 (1985).
Clark, S.S. et al. Expression of a distinctive BCR-ABL oncogene in Ph1-positive acute lymphocytic leukemia (ALL). Science 239, 775–777 (1988).
Van Etten, R.A. in Leukemia: Advances in Treatment and Research (eds Freireich, E.J. & Kantarjian, H.) 294–325 (Kluwer Academic, Norwood, 1993).
Heisterkamp, N., et al. Acute leukaemia in bcr/abl transgenic mice. Nature 344, 251–253 (1990).
Heisterkamp, N., Jenster, G., Kioussis, D., Pattengale, P.K. & Groffen, J. Human bcr-abl gene has a lethal effect on embryogenesis. Transgenic Res. 1, 45–53 (1991).
Honda, H., et al. Expression of p210bcr/abl by metallothionein promoter induced T-cell leukemia in transgenic mice. Blood 85, 2853–2861 (1995).
Honda, H., et al. Development of acute lymphoblastic leukemia and myeloproliferative disorder in transgenic mice expressing p210(bcr/abl): a novel transgenic model for human Ph1-positive leukemias. Blood 91, 2067–2075 (1998).
Castellanos, A., et al. A BCR-ABL(p190) fusion gene made by homologous recombination causes B-cell acute lymphoblastic leukemias in chimeric mice with independence of the endogenous bcr product. Blood 90, 2168–2174 (1997).
Furth, P.A., et al. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc. Natl Acad. Sci. USA 91, 9302–9306 (1994).
Hennighausen, L., Wall, R.J., Tillmann, U., Li, M. & Furth, P.A. Conditional gene expression in secretory tissues and skin of transgenic mice using the MMTV-LTR and the tetracycline responsive system. J. Cell. Biochem. 59, 463–472 (1995).
Hardy, R.R., Carmack, C.E., Shinton, S.A., Kemp, J.D. & Hayakawa, K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 173, 1213–1225 (1991).
Radomska, H.S. et al. Multiple control elements are required for expression of the human CD34 gene. Gene 222, 305–318 (1998).
Van Etten, R.A. in Medical Management of Chronic Myelogenous Leukemia (eds Talpaz, M. & Kantarjian, H.) 77–101 (Marcel Dekker, New York, 1998).
McGahon, A., et al. BCR-ABL maintains resistance of chronic myelogenous leukemia cells to apoptotic cell death. Blood 83, 1179–1187 (1994).
Bedi, A., Zehnbauer, B.A., Barber, J.P., Sharkis, S.J. & Jones, R.J. Inhibition of apoptosis by BCR-ABL in chronic myeloid leukemia. Blood 83, 2038–2044 (1994).
Chen, Y.Y. & Rosenberg, N. Lymphoid cells transformed by Abelson virus require the v-abl protein-tyrosine kinase only during early G1. Proc. Natl Acad. Sci. USA 89, 6683–6687 (1992).
Ewald, D., et al. Time-sensitive reversal of hyperplasia in transgenic mice expressing SV40 T antigen. Science 273, 1384–1386 (1996).
Druker, B.J., et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nature Med. 2, 561–566 (1996).
Daley, G.Q., Van Etten, R.A. & Baltimore, D. Blast crisis in a murine model of chronic myelogenous leukemia. Proc. Natl Acad. Sci. USA 88, 11335–11338 (1991).
Tanaka, M. & Herr, W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell 60, 375–386 (1990).
Cross, N.C., et al. Minimal residual disease after allogeneic bone marrow transplantation for chronic myeloid leukaemia in first chronic phase: correlations with acute graft-versus-host disease and relapse. Br. J. Haematol. 84, 67–74 (1993).
Kraig, E., et al. T and B cells that recognized the same antigen do not transcribe similar heavy chain variable region gene segments. J. Exp. Med. 158, 192–209 (1983).
Leder, A., Pattengale, P.K., Kuo, A., Stewart, T.A. & Leder, P. Consequences of widespread deregulation of the c-myc gene in transgenic mice: multiple neoplasms and normal development. Cell 45, 485–495 (1986).
Ilaria, R.L. Jr & Van Etten, R.A. The SH2 domain of P210BCR/ABL is not required for the transformation of hematopoietic factor-dependent cells. Blood 86, 3897–3904 (1995).
Gavrieli, Y., Sherman, Y. & Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell. Biol. 119, 493–501 (1992).
Acknowledgements
We thank M. Fenyus for assistance in animal care; L. Hennighausen for providing the MMTV-tTA strain; J. Lawitts of the BIDMC transgenic facility for expert rederivation and generation of transgenic lines; S. Takamatsu for assistance with photography; M. Singleton for assistance with preparation of the manuscript; and J.D. Griffin, G. Gilliland, D.-E. Zhang, K.P. Lu, C. Carpenter and L.K. Clayton for useful discussions. This work was supported by grants from the NIH to D.G.T., research grants from the NIH and the Leukemia Society of America to R.A.V. and from the Deutsche Forschungsgemeinschaft to C.S.H.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huettner, C., Zhang, P., Van Etten, R. et al. Reversibility of acute B-cell leukaemia induced by BCR–ABL1. Nat Genet 24, 57–60 (2000). https://doi.org/10.1038/71691
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/71691
This article is cited by
-
Targeting IL-17A enhances imatinib efficacy in Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia
Nature Communications (2024)
-
Activation of recombinational repair in Ewing sarcoma cells carrying EWS-FLI1 fusion gene by chromosome translocation
Scientific Reports (2022)
-
Metabolic convergence on lipogenesis in RAS, BCR-ABL, and MYC-driven lymphoid malignancies
Cancer & Metabolism (2021)
-
Syngeneic leukemia models using lentiviral transgenics
Cell Death & Disease (2021)
-
The lysophospholipase D enzyme Gdpd3 is required to maintain chronic myelogenous leukaemia stem cells
Nature Communications (2020)