Abstract
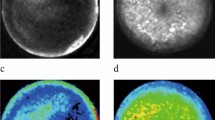
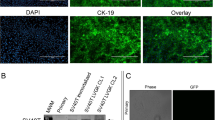
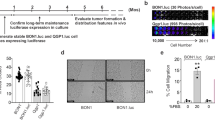
Syrian hamster is a practical animal model for studying the systemic effects of oncolytic vectors derived from adenovirus serotype 5 (Ad5). Ad5 replicates well in Syrian hamster tissues, and Syrian hamster cell lines are available that are known to support Ad5 replication. In this study, we established four new Syrian hamster cell lines from transplantable pancreatic, renal, hepatic and lung tumors. The pancreatic cell line (SHPC6) and the renal cell line were highly permissive for Ad5 replication. The SHPC6 cell line formed disseminated intraperitoneal tumors when cells were injected into the peritoneal cavity. INGN 007, an oncolytic Ad5-based vector, completely reversed the growth of disseminated intraperitoneal SHPC6 tumor nodules following intraperitoneal injection of the vector, leading to 100% survival of the treated animals. SHPC6 cells also formed subcutaneous tumors, whose growth was suppressed by INGN 007 following intratumoral injection. INGN 007 replicated in both the intraperitoneal and subcutaneous SHPC6 tumors. Following intraperitoneal injection, INGN 007 did not replicate in the livers of hamsters with intraperitoneal SHPC6 tumors, and was not hepatotoxic. These studies suggest that the SHPC6 cell line may be useful as a model for disseminated pancreatic cancer, and that INGN 007 may be a safe and effective vector to treat these tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cattaneo R, Miest T, Shashkova EV, Barry MA . Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol 2008; 6: 529–540.
Shirakawa T . The current status of adenovirus-based cancer gene therapy. Mol Cells 2008; 25: 462–466.
Lin E, Nemunaitis J . Oncolytic viral therapies. Cancer Gene Ther 2004; 11: 643–664.
Nemunaitis J, Vorhies JS, Pappen B, Senzer N . 10-year follow-up of gene-modified adenoviral-based therapy in 146 non-small-cell lung cancer patients. Cancer Gene Ther 2007; 14: 762–763.
Reid T, Warren R, Kirn D . Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther 2002; 9: 979–986.
Doronin K, Toth K, Kuppuswamy M, Ward P, Tollefson AE, Wold WSM . Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J Virol 2000; 74: 6147–6155.
Doronin K, Kuppuswamy M, Toth K, Tollefson AE, Krajcsi P, Krougliak V et al. Tissue-specific, tumor-selective, replication-competent adenovirus vector for cancer gene therapy. J Virol 2001; 75: 3314–3324.
Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE, Wold WSM . Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology 2003; 305: 378–387.
Kuppuswamy M, Spencer JF, Doronin K, Tollefson AE, Wold WS, Toth K . Oncolytic adenovirus that overproduces ADP and replicates selectively in tumors due to hTERT promoter-regulated E4 gene expression. Gene Therapy 2005; 12: 1608–1617.
Toth K, Djeha H, Ying BL, Tollefson AE, Kuppuswamy M, Doronin K et al. An oncolytic adenovirus vector combining enhanced cell-to-cell spreading, mediated by the ADP cytolytic protein, with selective replication in cancer cells with deregulated Wnt signaling. Cancer Res 2004; 64: 3638–3644.
Tollefson AE, Ryerse JS, Scaria A, Hermiston TW, Wold WSM . The E3-11.6kDa adenovirus death protein (ADP) is required for efficient cell death: characterization of cells infected with adp mutants. Virology 1996; 220: 152–162.
Tollefson AE, Scaria A, Hermiston TW, Ryerse JS, Wold LJ, Wold WSM . The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J Virol 1996; 70: 2296–2306.
Lichtenstein DL, Spencer JF, Doronin K, Patra D, Meyer J, Shashkova EV et al. An acute toxicology study with INGN 007, an oncolytic adenovirus vector, in mice and permissive Syrian hamsters; comparisons with wild-type Ad5 and a replication-defective adenovirus vector. Cancer Gene Ther 2009; doi:10.1038/cgt.2009.5.
Thomas MA, Spencer JF, La Regina MC, Dhar D, Tollefson AE, Toth K et al. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res 2006; 66: 1270–1276.
Toth K, Tarakanova V, Doronin K, Ward P, Kuppuswamy M, Locke JL et al. Radiation increases the activity of oncolytic adenovirus cancer gene therapy vectors that overexpress the ADP (E3-11.6K) protein. Cancer Gene Ther 2003; 10: 193–200.
Toth K, Spencer JF Tollefson AE, Kuppuswamy M, Doronin K, Lichtenstein DL, La Regina MC et al. Cotton rat tumor model for the evaluation of oncolytic adenoviruses. Hum Gene Ther 2005; 16: 139–146.
Ying B, Toth K, Spencer JF, Meyer J, Tollefson AE, Patra D et al. INGN 007, an oncolytic adenovirus vector, replicates in Syrian hamsters but not mice: comparison of biodistribution studies. Cancer Gene Ther 2009; doi:10.1038/cgt.2009.6.
Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips N, Wold WSM . Immunosuppression enhances oncolytic adenovirus replication, anti tumor efficacy in the Syrian hamster model. Mol Ther 2008; 16: 1665–1673.
Alemany R . Cancer selective adenoviruses. Mol Aspects Med 2007; 28: 42–58.
Hedley SJ, Chen J, Mountz JD, Li J, Curiel DT, Korokhov N et al. Targeted, shielded adenovectors for cancer therapy. Cancer Immunol Immunother 2006; 55: 1412–1419.
Nettelbeck DM . Cellular genetic tools to control oncolytic adenoviruses for virotherapy of cancer. J Mol Med 2008; 86: 363–377.
Ribacka C, Hemminki A . Virotherapy as an approach against cancer stem cells. Curr Gene Ther 2008; 8: 88–96.
Duncan SJ, Gordon FC, Gregory DW, McPhie JL, Postlethwaite R, White R et al. Infection of mouse liver by human adenovirus type 5. J Gen Virol 1978; 40: 45–61.
Ginsberg HS, Moldawer LL, Sehgal PB, Redington M, Kilian PL, Chanock RM et al. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci USA 1991; 88: 1651–1655.
Oualikene W, Gonin P, Eloit M . Short, long term dissemination of deletion mutants of adenovirus in permissive (cotton rat) and non-permissive (mouse) species. J Gen Virol 1994; 75: 2765–2768.
Prince GA, Porter DD, Jenson AB, Horswood RL, Chanock RM, Ginsberg HS . Pathogenesis of adenovirus type 5 pneumonia in cotton rats (Sigmodon hispidus). J Virol 1993; 67: 101–111.
Jogler C, Hoffmann D, Theegarten D, Grunwald T, Uberla K, Wildner O . Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species. J Virol 2006; 80: 3549–3558.
Ternovoi VV, Le LP, Belousova N, Smith BF, Siegal GP, Curiel DT . Productive replication of human adenovirus type 5 in canine cells. J Virol 2005; 79: 1308–1311.
Bortolanza S, Alzuguren P, Bunuales M, Qian C, Prieto J, Hernandez-Alcoceba R . Human adenovirus replicates in immunocompetent models of pancreatic cancer in Syrian hamsters. Hum Gene Ther 2007; 18: 681–690.
Shashkova EV, Spencer JF, Wold WSM, Doronin K . Targeting interferon-alpha increases antitumor efficacy and reduces hepatotoxicity of E1A-mutated spread-enhanced oncolytic adenovirus. Mol Ther 2007; 15: 598–607.
Thomas MA, Spencer JF, Wold WSM . Use of the Syrian hamster as an animal model for oncolytic adenovirus vectors. Methods Mol Med 2007; 130: 169–183.
Toth K, Spencer JF, Dhar D, Sagartz JE, Buller RM, Painter GR et al. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc Natl Acad Sci USA 2008; 105: 7293–7297.
Hjorth RN, Bonde GM, Pierzchala WA, Vernon SK, Wiener FP, Levner MH et al. A new hamster model for adenoviral vaccination. Arch Virol 1988; 100: 279–283.
Dhar D, Spencer JF, Toth K, Wold WSM . Effect of pre-existing immunity on oncolytic adenovirus INGN 007 vector anti-tumor efficacy in immunocompetent and immunosuppressed Syrian hamsters. J Virol 2009; 83: 2130–2139.
Zarubaev VV, Slita AV, Sukhinin VP, Nosach LN, Dyachenko NS, Povnitsa OY et al. Effect of 6-azacytidine on the course of experimental adenoviral infection in newborn Syrian hamsters. J Chemother 2007; 19: 44–51.
Tollefson AE, Kuppuswamy M, Shashkova EV, Doronin K, Wold WSM . Preparation and titration of CsCl-banded adenovirus stocks. Methods Mol Med 2007; 130: 223–235.
Thomas MA, Spencer JF, Wold WSM . The use of the Syrian hamster as an animal model for oncolytic adenovirus Vectors. In: Tollefson A.E. and Wold W.S.M., eds. Adenovirus Methods and Protocols. Second, Humana Press, Totowa, NJ. 2005.
Toth K, Spencer JF, Wold WSM . Immunocompetent, semi-permissive cotton rat tumor model for the evaluation of oncolytic adenoviruses. In: Tollefson A. E. and Wold W. S. M., eds Adenovirus Methods and Protocols. Second, Humana Press, Totowa, NJ. 2007: 157–168.
Kirkman H, Chesterman FC . Additional data on transplanted tumours of the golden hamster. Prog Exp Tumor Res 1972; 16: 580–621.
Yonemori K, Okusaka T, Ueno H, Morizane C, Takesako Y, Ikeda M . FP therapy for controlling malignant ascites in advanced pancreatic cancer patients. Hepatogastroenterology 2007; 54: 2383–2386.
Zervos EE, Osborne D, Boe BA, Luzardo G, Goldin SB, Rosemurgy AS . Prognostic significance of new onset ascites in patients with pancreatic cancer. World J Surg Oncol 2006; 4: 16.
Miura Y, Ohnami S, Yoshida K, Ohashi M, Nakano M, Ohnami S et al. Intraperitoneal injection of adenovirus expressing antisense K-ras RNA suppresses peritoneal dissemination of hamster syngeneic pancreatic cancer without systemic toxicity. Cancer Lett 2005; 218: 53–62.
Rigg AS, Lemoine NR . Adenoviral delivery of TIMP1 or TIMP2 can modify the invasive behavior of pancreatic cancer and can have a significant antitumor effect in vivo. Cancer Gene Ther 2001; 8: 869–878.
Yamamura S, Onda M, Uchida E . Two types of peritoneal dissemination of pancreatic cancer cells in a hamster model. J Nippon Med Sch 1999; 66: 253–261.
Kasuya H, Takeda S, Nomoto S, Nakao A . The potential of oncolytic virus therapy for pancreatic cancer. Cancer Gene Ther 2005; 12: 725–736.
Ramirez PJ, Vickers SM . Adenoviral gene therapy for pancreatic adenocarcinoma. Curr Surg 2004; 61: 351–358.
Freytag SO, Barton KN, Brown SL, Narra V, Zhang Y, Tyson D et al. Replication-competent adenovirus-mediated suicide gene therapy with radiation in a preclinical model of pancreatic cancer. Mol Ther 2007; 15: 1600–1606.
Vasey PA, Shulman LN, Campos S, Davis J, Gore M, Johnston S et al. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J Clin Oncol 2002; 20: 1562–1569.
Iki K, Pour PM . Expression of Oct4, a stem cell marker, in the hamster pancreatic cancer model. Pancreatology 2006; 6: 406–413.
Crowell PL, Schmidt CM, Yip-Schneider MT, Savage JJ, Hertzler DA, Cummings WO . Cyclooxygenase-2 expression in hamster and human pancreatic neoplasia. Neoplasia 2006; 8: 437–445.
Acknowledgements
This research was supported by grants CA118022 and CA108335 to W.S.M.W. from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spencer, J., Sagartz, J., Wold, W. et al. New pancreatic carcinoma model for studying oncolytic adenoviruses in the permissive Syrian hamster. Cancer Gene Ther 16, 912–922 (2009). https://doi.org/10.1038/cgt.2009.36
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2009.36
This article is cited by
-
Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent
Nature Communications (2017)
-
Evaluation of apoptogenic adenovirus type 5 oncolytic vectors in a Syrian hamster head and neck cancer model
Cancer Gene Therapy (2014)
-
The role of cyclophosphamide in enhancing antitumor efficacy of an adenovirus oncolytic vector in subcutaneous Syrian hamster tumors
Cancer Gene Therapy (2013)
-
The effects of radiation on antitumor efficacy of an oncolytic adenovirus vector in the Syrian hamster model
Cancer Gene Therapy (2013)
-
A fully replication-competent adenovirus vector with enhanced oncolytic properties
Cancer Gene Therapy (2010)