Abstract
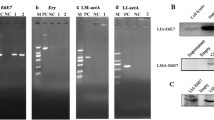
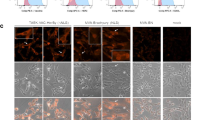
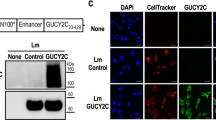
A chimeric human Her2/neu gene (ChHer2) harboring most of the known major histocompatibility complex class I epitopes of the HER2/neu oncogene was expressed as a fusion protein to a non-hemolytic fragment of listeriolysin O (LLO), by the highly attenuated Listeria vector LmddA, which lacks antibiotic selection markers and the ability to spread from cell-to-cell. This construct (ADXS31–164) was tested for immunogenicity and anti-tumor effects in mice. Despite being highly attenuated, ADXS31–164 proved to be efficacious in breaking immune tolerance toward the HER2/neu self-antigen. ADXS31–164 elicited strong T-cell immune responses in experimental animals. In tumors, ADXS31–164 caused a reduction in regulatory T cells (Treg) accompanied by an increase in the CD8+/Treg ratio. Comparison of this vaccine with the conventional antibiotic resistant Listeria vector (Lm-LLO-ChHer2) shows that ADXS31–164 is more efficacious in delaying tumor growth in Her2/neu transgenic animals. Because of its well-defined attenuation mechanism and independence from antibiotic selection markers, ADXS31–164 is potentially more suitable for human use. These results support the future clinical development of this vaccine for the treatment of HER2/neu-overexpressing malignancies, such as breast, colorectal and pancreatic cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Reynolds A . Stereotactic breast biopsy: a review. Radiol Technol 2009; 80: 447M–464M.
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244: 707–712.
Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C . Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 1995; 378: 394–398.
Singh R, Dominiecki ME, Jaffee EM, Paterson Y . Fusion to Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J Immunol 2005; 175: 3663–3673.
Seavey MM, Pan ZK, Maciag PC, Wallecha A, Rivera S, Paterson Y et al. A novel human Her-2/neu chimeric molecule expressed by Listeria monocytogenes can elicit potent HLA-A2 restricted CD8-positive T cell responses and impact the growth and spread of Her-2/neu-positive breast tumors. Clin Cancer Res 2009; 15: 924–932.
Wallecha A, Maciag PC, Rivera S, Paterson Y, Shahabi V . Construction and characterization of an attenuated Listeria monocytogenes strain for clinical use in cancer immunotherapy. Clin Vaccine Immunol 2009; 16: 96–103.
Reilly RT, Gottlieb MB, Ercolini AM, Machiels JP, Kane CE, Okoye FI et al. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res 2000; 60: 3569–3576.
Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y . Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol 2001; 167: 6471–6479.
Peters C, Paterson Y . Enhancing the immunogenicity of bioengineered Listeria monocytogenes by passaging through live animal hosts. Vaccine 2003; 21: 1187–1194.
Shahabi V, Reyes-Reyes M, Wallecha A, Rivera S, Paterson Y, Maciag P . Development of a Listeria monocytogenes based vaccine against prostate cancer. Cancer Immunol Immunother 2008; 57: 1301–1313.
Fontenot JD, Gavin MA, Rudensky AY . Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4: 330–336.
Singh R, Paterson Y . Immunoediting sculpts tumor epitopes during immunotherapy. Cancer Res 2007; 67: 1887–1892.
Kawashima I, Tsai V, Southwood S, Takesako K, Sette A, Celis E . Identification of HLA-A3-restricted cytotoxic T lymphocyte epitopes from carcinoembryonic antigen and HER-2/neu by primary in vitro immunization with peptide-pulsed dendritic cells. Cancer Res 1999; 59: 431–435.
Rongcun Y, Salazar-Onfray F, Charo J, Malmberg KJ, Evrin K, Maes H et al. Identification of new HER2/neu-derived peptide epitopes that can elicit specific CTL against autologous and allogeneic carcinomas and melanomas. J Immunol 1999; 163: 1037–1044.
Verch T, Pan ZK, Paterson Y . Listeria monocytogenes-based antibiotic resistance gene-free antigen delivery system applicable to other bacterial vectors and DNA vaccines. Infect Immun 2004; 72: 6418–6425.
Pegram M . Can we circumvent resistance to ErbB2-targeted agents by targeting novel pathways? Clin Breast Cancer 2008; 8 (Suppl 3): S121–S130.
Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B et al. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res 2008; 14: 2413–2420.
Li Z, Zhao X, Zhou C, Gu B, Frankel FR . A truncated Bacillus subtilis dal gene with a 3′ ssrA gene tag regulates the growth and virulence of racemase-deficient Listeria monocytogenes. Microbiology 2006; 152 (Part 10): 3091–3102.
Thompson RJ, Bouwer HG, Portnoy DA, Frankel FR . Pathogenicity and immunogenicity of a Listeria monocytogenes strain that requires D-alanine for growth. Infect Immun 1998; 66: 3552–3561.
Nitcheu-Tefit J, Dai MS, Critchley-Thorne RJ, Ramirez-Jimenez F, Xu M, Conchon S et al. Listeriolysin O expressed in a bacterial vaccine suppresses CD4+CD25high regulatory T cell function in vivo. J Immunol 2007; 179: 1532–1541.
Nishikawa H, Tsuji T, Jager E, Briones G, Ritter G, Old LJ et al. Induction of regulatory T cell-resistant helper CD4+ T cells by bacterial vector. Blood 2008; 111: 1404–1412.
Kim SH, Castro F, Paterson Y, Gravekamp C . High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res 2009; 69: 5860–5866.
Wallecha A, Carroll KD, Maciag PC, Rivera S, Shahabi V, Paterson Y . Multiple effector mechanisms induced by recombinant Listeria monocytogenes anticancer immunotherapeutics. Adv Appl Microbiol 2009; 66: 1–27.
Algarra I, Garcia-Lora A, Cabrera T, Ruiz-Cabello F, Garrido F . The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol Immunother 2004; 53: 904–910.
Campoli M, Chang CC, Ferrone S . HLA class I antigen loss, tumor immune escape and immune selection. Vaccine 2002; 20 (Suppl 4): A40–A45.
Griffioen AW, Damen CA, Martinotti S, Blijham GH, Groenewegen G . Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res 1996; 56: 1111–1117.
Brown KA . Factors modifying the migration of lymphocytes across the blood-brain barrier. Int Immunopharmacol 2001; 1: 2043–2062.
Prins RM, Bruhn KW, Craft N, Lin JW, Kim CH, Odesa SK et al. Central nervous system tumor immunity generated by a recombinant listeria monocytogenes vaccine targeting tyrosinase related protein-2 and real-time imaging of intracranial tumor burden. Neurosurgery 2006; 58: 169–178; discussion 169–178.
Maciag PC, Radulovic S, Rothman J . The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a phase i safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine 2009; 27: 3975–3983.
Acknowledgements
The imaging data presented here was performed at the Optical Imaging Core (supported in part by NIH Grant CA105008) of the Small Animal Imaging Facility at the University of Pennsylvania. We are also grateful to Dr Yingqui Yvette Liu and Dr Wafik S El-Deiry for their advice and assistance on the part of the optical imaging study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Advaxis Inc. is a for-profit vaccine and therapeutic company that has licensed or has an option to license all patents from the University of Pennsylvania that concern the use of Lms or listerial products as vaccines. The following authors have a financial interest in Advaxis Inc., and thus may have a conflict of interest: Drs PC Maciag, A Wallecha, S Rivera and V Shahabi. Dr MM Seavey declares no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Cancer Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Shahabi, V., Seavey, M., Maciag, P. et al. Development of a live and highly attenuated Listeria monocytogenes-based vaccine for the treatment of Her2/neu-overexpressing cancers in human. Cancer Gene Ther 18, 53–62 (2011). https://doi.org/10.1038/cgt.2010.48
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2010.48
Keywords
This article is cited by
-
Bacterial Peptides and Bacteriocins as Novel Treatment for Prostate Cancer
International Journal of Peptide Research and Therapeutics (2023)
-
The bacterial instrument as a promising therapy for colon cancer
International Journal of Colorectal Disease (2020)
-
White paper on microbial anti-cancer therapy and prevention
Journal for ImmunoTherapy of Cancer (2018)
-
Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy
Gynecologic Oncology Research and Practice (2017)
-
Agonist anti-GITR antibody significantly enhances the therapeutic efficacy of Listeria monocytogenes-based immunotherapy
Journal for ImmunoTherapy of Cancer (2017)