Abstract
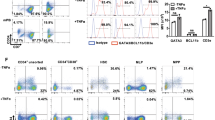
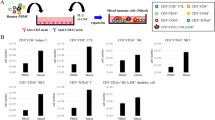
We previously reported a method to generate dendritic cell (DC)-like antigen-presenting cells (APC) from human induced pluripotent stem (iPS) cells. However, the method is relatively complicated and laborious. In the current study, we attempted to establish a method through which we could obtain a large number of functional APC with a simple procedure. We transduced iPS cell-derived CD11b+ myeloid cells with genes associated with proliferative or anti-senescence effects, enabling the cells to propagate for more than 4 months in a macrophage colony-stimulating factor (M-CSF)-dependent manner while retaining their capacity to differentiate into functional APC. We named these iPS cell-derived proliferating myeloid cells ‘iPS-ML’, and the iPS-ML-derived APC ‘ML-DC’. In addition, we generated TAP2-deficient iPS cell clones by zinc finger nuclease-aided targeted gene disruption. TAP2-deficient iPS cells and iPS-ML avoided recognition by pre-activated allo-reactive CD8+ T cells. TAP2-deficient ML-DC expressing exogenously introduced HLA-A2 genes stimulated HLA-A2-restricted MART-1-specific CD8+ T cells obtained from HLA-A2-positive allogeneic donors, resulting in generation of MART-1-specific cytotoxic T lymphocyte (CTL) lines. TAP-deficient iPS-ML introduced with various HLA class I genes may serve as an unlimited source of APC for vaccination therapy. If administered into allogeneic patients, ML-DC with appropriate genetic modifications may survive long enough to stimulate antigen-specific CTL and, after that, be completely eliminated. Based on the present study, we propose an APC-producing system that is simple, safe and applicable to all patients irrespective of their HLA types.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Abbreviations
- APC:
-
antigen-presenting cells
- DC:
-
dendritic cells
- ES cell:
-
embryonic stem cell
- iPS cell:
-
induced pluripotent stem cell
- iPS-MC:
-
iPS cell-derived myeloid cells
- iPS-ML:
-
iPS cell-derived myeloid cell line
- ML-DC:
-
iPS-ML-derived dendritic cell-like APC.
References
Eksioglu EA, Eisen S, Reddy V . Dendritic cells as therapeutic agents against cancer. Front Biosci 2010; 15: 321–347.
Robson NC, Hoves S, Maraskovsky E, Schnurr M . Presentation of tumour antigens by dendritic cells and challenges faced. Curr Opin Immunol 2010; 22: 137–144.
Schuler G . Dendritic cells in cancer immunotherapy. Eur J Immunol 2010; 40: 2123–2130.
Takahashi K, Yamanaka S . Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126: 663–676.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131: 861–872.
Fairchild PJ, Brook FA, Gardner RL, Graca L, Strong V, Tone Y et al. Directed differentiation of dendritic cells from mouse embryonic stem cells. Curr Biol 2000; 10: 1515–1518.
Senju S, Hirata S, Matsuyoshi H, Masuda M, Uemura Y, Araki K et al. Generation and genetic modification of dendritic cells derived from mouse embryonic stem cells. Blood 2003; 101: 3501–3508.
Senju S, Haruta M, Matsunaga Y, Fukushima S, Ikeda T, Takahashi K et al. Characterization of dendritic cells and macrophages generated by directed differentiation from mouse induced pluripotent stem cells. Stem Cells 2009; 27: 1021–1031.
Zhan X, Dravid G, Ye Z, Hammond H, Shamblott M, Gearhart J et al. Functional antigen-presenting leucocytes derived from human embryonic stem cells in vitro. Lancet 2004; 364: 163–171.
Slukvin II, Vodyanik MA, Thomson JA, Gumenyuk ME, Choi KD . Directed differentiation of human embryonic stem cells into functional dendritic cells through the myeloid pathway. J Immunol 2006; 176: 2924–2932.
Su Z, Frye C, Bae KM, Kelley V, Vieweg J . Differentiation of human embryonic stem cells into immunostimulatory dendritic cells under feeder-free culture conditions. Clin Cancer Res 2008; 14: 6207–6217.
Senju S, Suemori H, Zembutsu H, Uemura Y, Hirata S, Fukuma D et al. Genetically manipulated human embryonic stem cell-derived dendritic cells with immune regulatory function. Stem Cells 2007; 25: 2720–2729.
Tseng SY, Nishimoto KP, Silk KM, Majumdar AS, Dawes GN, Waldmann H et al. Generation of immunogenic dendritic cells from human embryonic stem cells without serum and feeder cells. Regen Med 2009; 4: 513–526.
Fukushima S, Hirata S, Motomura Y, Fukuma D, Matsunaga Y, Ikuta Y et al. Multiple antigen-targeted immunotherapy with alpha-galactosylceramide-loaded and genetically engineered dendritic cells derived from embryonic stem cells. J Immunother 2009; 32: 219–231.
Matsuyoshi H, Senju S, Hirata S, Yoshitake Y, Uemura Y, Nishimura Y . Enhanced priming of antigen-specific CTLs in vivo by embryonic stem cell-derived dendritic cells expressing chemokine along with antigenic protein: application to antitumor vaccination. J Immunol 2004; 172: 776–786.
Motomura Y, Senju S, Nakatsura T, Matsuyoshi H, Hirata S, Monji M et al. Embryonic stem cell-derived dendritic cells expressing glypican-3, a recently identified oncofetal antigen, induce protective immunity against highly metastatic mouse melanoma, B16-F10. Cancer Res 2006; 66: 2414–2422.
Senju S, Haruta M, Matsumura K, Matsunaga Y, Fukushima S, Ikeda T et al. Generation of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Therapy 2011; 18: 874–883.
Choi KD, Vodyanik M, Slukvin II . Hematopoietic differentiation and production of mature myeloid cells from human pluripotent stem cells. Nat Protoc 2011; 6: 296–313.
Nakahara S, Tsunoda T, Baba T, Asabe S, Tahara H . Dendritic cells stimulated with a bacterial product, OK-432, efficiently induce cytotoxic T lymphocytes specific to tumor rejection peptide. Cancer Res 2003; 63: 4112–4118.
Tabata H, Kanai T, Yoshizumi H, Nishiyama S, Fujimoto S, Matsuda I et al. Characterization of self-glutamic acid decarboxylase 65-reactive CD4+ T-cell clones established from Japanese patients with insulin-dependent diabetes mellitus. Hum Immunol 1998; 59: 549–560.
Neefjes JJ, Momburg F, Hammerling GJ . Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science 1993; 261: 769–771.
Zwaka TP, Thomson JA . Homologous recombination in human embryonic stem cells. Nat Biotechnol 2003; 21: 319–321.
Kim YG, Cha J, Chandrasegaran S . Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci U S A 1996; 93: 1156–1160.
Bacik I, Cox JH, Anderson R, Yewdell JW, Bennink JR . TAP (transporter associated with antigen processing)-independent presentation of endogenously synthesized peptides is enhanced by endoplasmic reticulum insertion sequences located at the amino- but not carboxyl-terminus of the peptide. J Immunol 1994; 152: 381–387.
Fukuma D, Matsuyoshi H, Hirata S, Kurisaki A, Motomura Y, Yoshitake Y et al. Cancer prevention with semi-allogeneic ES cell-derived dendritic cells. Biochem Biophys Res Commun 2005; 335: 5–13.
Wells JW, Cowled CJ, Darling D, Guinn BA, Farzaneh F, Noble A et al. Semi-allogeneic dendritic cells can induce antigen-specific T-cell activation, which is not enhanced by concurrent alloreactivity. Cancer Immunol Immunother 2007; 56: 1861–1873.
Loyer V, Fontaine P, Pion S, Hetu F, Roy DC, Perreault C . The in vivo fate of APCs displaying minor H antigen and/or MHC differences is regulated by CTLs specific for immunodominant class I-associated epitopes. J Immunol 1999; 163: 6462–6467.
Hermans IF, Ritchie DS, Yang J, Roberts JM, Ronchese F . CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J Immunol 2000; 164: 3095–3101.
Matsunaga Y, Fukuma D, Hirata S, Fukushima S, Haruta M, Ikeda T et al. Activation of antigen-specific cytotoxic T lymphocytes by beta2-microglobulin or TAP1 gene disruption and the introduction of recipient-matched MHC class I gene in allogeneic embryonic stem cell-derived dendritic cells. J Immunol 2008; 181: 6635–6643.
Lee S, Schmitt CA, Reimann M . The Myc/macrophage tango: oncogene-induced senescence, Myc style. Semin Cancer Biol 2011; 21: 377–384.
Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M . The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 1999; 397: 164–168.
Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev 2007; 21: 525–530.
Kamminga LM, Bystrykh LV, de Boer A, Houwer S, Douma J, Weersing E et al. The Polycomb group gene Ezh2 prevents hematopoietic stem cell exhaustion. Blood 2006; 107: 2170–2179.
Weinberg JB, Haney AF, Xu FJ, Ramakrishnan S . Peritoneal fluid and plasma levels of human macrophage colony-stimulating factor in relation to peritoneal fluid macrophage content. Blood 1991; 78: 513–516.
Hovden AO, Karlsen M, Jonsson R, Aarstad HJ, Appel S . Maturation of monocyte derived dendritic cells with OK432 boosts IL-12p70 secretion and conveys strong T-cell responses. BMC Immunol 2011; 12: 2.
Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM . Development of a self-inactivating lentivirus vector. J Virol 1998; 72: 8150–8157.
Kuzushima K, Hayashi N, Kimura H, Tsurumi T . Efficient identification of HLA-A*2402-restricted cytomegalovirus-specific CD8(+) T-cell epitopes by a computer algorithm and an enzyme-linked immunospot assay. Blood 2001; 98: 1872–1881.
Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med 1994; 180: 347–352.
Acknowledgements
The cDNA clones for MDM2, BMI1, and EZH2 and 293T cells were provided by RIKEN BRC, which is participating in the National Bio-Resources Project of the MEXT, Japan. The pCSII-EF, pCMV-VSV-G-RSV-Rev and pCAG-HIVgp constructs were kindly provided by Dr H Miyoshi (RIKEN BioResource Center). This work was supported in part by Grants-in-Aid Nos 18014023, 19591172 and 19059012 from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, Research Grant for Intractable Diseases from Ministry of Health and Welfare, Japan and grants from Japan Science and Technology Agency (JST).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Haruta, M., Tomita, Y., Yuno, A. et al. TAP-deficient human iPS cell-derived myeloid cell lines as unlimited cell source for dendritic cell-like antigen-presenting cells. Gene Ther 20, 504–513 (2013). https://doi.org/10.1038/gt.2012.59
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2012.59
Keywords
This article is cited by
-
Reevaluation of antibody-dependent enhancement of infection in anti-SARS-CoV-2 therapeutic antibodies and mRNA-vaccine antisera using FcR- and ACE2-positive cells
Scientific Reports (2022)
-
The potential of COVID-19 patients’ sera to cause antibody-dependent enhancement of infection and IL-6 production
Scientific Reports (2021)
-
Lysosomal membrane permeabilization causes secretion of IL-1β in human vascular smooth muscle cells
Inflammation Research (2018)
-
Functional disruption of human leukocyte antigen II in human embryonic stem cell
Biological Research (2015)
-
Antigenically Modified Human Pluripotent Stem Cells Generate Antigen-Presenting Dendritic Cells
Scientific Reports (2015)