Abstract
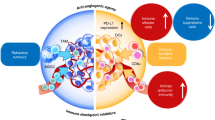
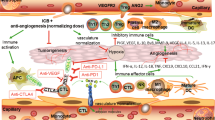
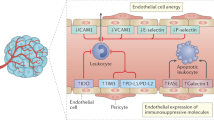
Much progress and significant therapeutic changes have been made in the field of tumor therapy in the past decades. Besides chemotherapy and radiotherapy, a special focus was laid on targeted therapies such as small molecule tyrosine kinase inhibitors (TKIs) and other immunomodulatory drugs, which have become standard therapies and important combination partners in a variety of malignancies. In contrast to the widely established use of these often anti-angiogenic drugs, many functional molecular mechanisms are yet not completely understood. Recent analyses focused not only on their direct anti-tumor responses, but also on their influence on tumor microenvironment, as well as on their effects on malignant and healthy cells. Different anti-angiogenic compounds targeting the vascular endothelial growth factor (VEGF) or platelet-derived growth factor pathways seem to be capable of modulating immune responses, in a positive, as well as apparently harmful manner. For an optimal clinical anti-cancer treatment, a better understanding of these immunomodulatory effects is necessary. Here we summarize recent reports on the immunomodulatory function of lately introduced clinically applied anti-angiogenic compounds, such as the humanized monoclonal antibody against VEGF bevacizumab, the small molecule TKIs sunitinib, sorafenib, imatinib, dasatinib, nilotinib and the proteasome inhibitor bortezomib.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Baselga J . Targeting tyrosine kinases in cancer: the second wave. Science 2006; 312: 1175–1178.
Osusky KL, Hallahan DE, Fu A, Ye F, Shyr Y, Geng L . The receptor tyrosine kinase inhibitor SU11248 impedes endothelial cell migration, tubule formation, and blood vessel formation in vivo, but has little effect on existing tumor vessels. Angiogenesis 2004; 7: 225–233.
Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J 2004; 18: 338–340.
Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL et al. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol 2002; 168: 4272–4276.
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10: 942–949.
Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E . Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 1999; 59: 3128–3133.
Shimizu J, Yamazaki S, Sakaguchi S . Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol 1999; 163: 5211–5218.
Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 2009; 15: 2148–2157.
Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res 2010; 70: 3526–3536.
Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res 2009; 69: 2514–2522.
Zhao W, Gu YH, Song R, Qu BQ, Xu Q . Sorafenib inhibits activation of human peripheral blood T cells by targeting LCK phosphorylation. Leukemia 2008; 22: 1226–1233.
Houben R, Voigt H, Noelke C, Hofmeister V, Becker JC, Schrama D . MAPK-independent impairment of T-cell responses by the multikinase inhibitor sorafenib. Mol Cancer Ther 2009; 8: 433–440.
Molhoek KR, McSkimming CC, Olson WC, Brautigan DL, Slingluff Jr CL . Apoptosis of CD4(+)CD25(high) T cells in response to Sirolimus requires activation of T cell receptor and is modulated by IL-2. Cancer Immunol Immunother 2009; 58: 867–876.
Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood 2008; 111: 5610–5620.
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003; 349: 427–434.
Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother 2008; 57: 1115–1124.
Alfaro C, Suarez N, Gonzalez A, Solao S, Erro L, Dubrot J et al. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br J Cancer 2009; 100: 1111–1119.
Zhang J, Silva T, Yarovinsky T, Manes TD, Tavakoli S, Nie L et al. VEGF blockade inhibits lymphocyte recruitment and ameliorates immune-mediated vascular remodeling. Circ Res 2010; 107: 408–417.
Wada J, Suzuki H, Fuchino R, Yamasaki A, Nagai S, Yanai K et al. The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions. Anticancer Res 2009; 29: 881–888.
Appel S, Rupf A, Weck MM, Schoor O, Brummendorf TH, Weinschenk T et al. Effects of imatinib on monocyte-derived dendritic cells are mediated by inhibition of nuclear factor-kappaB and Akt signaling pathways. Clin Cancer Res 2005; 11: 1928–1940.
Appel S, Balabanov S, Brummendorf TH, Brossart P . Effects of imatinib on normal hematopoiesis and immune activation. Stem Cells 2005; 23: 1082–1088.
Seggewiss R, Lore K, Greiner E, Magnusson MK, Price DA, Douek DC et al. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood 2005; 105: 2473–2479.
Taieb J, Maruyama K, Borg C, Terme M, Zitvogel L . Imatinib mesylate impairs Flt3L-mediated dendritic cell expansion and antitumor effects in vivo. Blood 2004; 103: 1966–1967.
Borg C, Terme M, Taieb J, Menard C, Flament C, Robert C et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest 2004; 114: 379–388.
Mohty M, Jourdan E, Mami NB, Vey N, Damaj G, Blaise D et al. Imatinib and plasmacytoid dendritic cell function in patients with chronic myeloid leukemia. Blood 2004; 103: 4666–4668.
Wang H, Cheng F, Cuenca A, Horna P, Zheng Z, Bhalla K et al. Imatinib mesylate (STI-571) enhances antigen-presenting cell function and overcomes tumor-induced CD4+ T-cell tolerance. Blood 2005; 105: 1135–1143.
Brauer KM, Werth D, von SK, Bringmann A, Kanz L, Grunebach F et al. BCR-ABL activity is critical for the immunogenicity of chronic myelogenous leukemia cells. Cancer Res 2007; 67: 5489–5497.
Schade AE, Schieven GL, Townsend R, Jankowska AM, Susulic V, Zhang R et al. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood 2008; 111: 1366–1377.
Blake S, Hughes TP, Mayrhofer G, Lyons AB . The Src/ABL kinase inhibitor dasatinib (BMS-354825) inhibits function of normal human T-lymphocytes in vitro. Clin Immunol 2008; 127: 330–339.
Fraser CK, Blake SJ, Diener KR, Lyons AB, Brown MP, Hughes TP et al. Dasatinib inhibits recombinant viral antigen-specific murine CD4+ and CD8+ T-cell responses and NK-cell cytolytic activity in vitro and in vivo. Exp Hematol 2009; 37: 256–265.
Weichsel R, Dix C, Wooldridge L, Clement M, Fenton-May A, Sewell AK et al. Profound inhibition of antigen-specific T-cell effector functions by dasatinib. Clin Cancer Res 2008; 14: 2484–2491.
Fei F, Yu Y, Schmitt A, Rojewski MT, Chen B, Greiner J et al. Dasatinib exerts an immunosuppressive effect on CD8+ T cells specific for viral and leukemia antigens. Exp Hematol 2008; 36: 1297–1308.
Fei F, Yu Y, Schmitt A, Rojewski MT, Chen B, Gotz M et al. Dasatinib inhibits the proliferation and function of CD4+CD25+ regulatory T cells. Br J Haematol 2009; 144: 195–205.
Mustjoki S, Ekblom M, Arstila TP, Dybedal I, Epling-Burnette PK, Guilhot F et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia 2009; 23: 1398–1405.
Kim DH, Kamel-Reid S, Chang H, Sutherland R, Jung CW, Kim HJ et al. Natural killer or natural killer/T cell lineage large granular lymphocytosis associated with dasatinib therapy for Philadelphia chromosome positive leukemia. Haematologica 2009; 94: 135–139.
Kreutzman A, Juvonen V, Kairisto V, Ekblom M, Stenke L, Seggewiss R et al. Mono/oligoclonal T- and NK-cells are common in chronic myeloid leukemia patients at diagnosis and expand during dasatinib therapy. Blood 2010; 116: 772–782.
Salih J, Hilpert J, Placke T, Grunebach F, Steinle A, Salih HR et al. The BCR/ABL-inhibitors imatinib, nilotinib, and dasatinib differentially affect NK cell reactivity. Int J Cancer 2010; 127: 2119–2128.
Chen J, Schmitt A, Chen B, Rojewski M, Rubeler V, Fei F et al. Nilotinib hampers the proliferation and function of CD8+ T lymphocytes through inhibition of T cell receptor signalling. J Cell Mol Med 2008; 12 (5B): 2107–2118.
Fei F, Yu Y, Schmitt A, Rojewski MT, Chen B, Greiner J et al. Effects of nilotinib on regulatory T cells: the dose matters. Mol Cancer 2010; 9: 22.
Nencioni A, Grunebach F, Patrone F, Ballestrero A, Brossart P . Proteasome inhibitors: antitumor effects and beyond. Leukemia 2007; 21: 30–36.
Nencioni A, Schwarzenberg K, Brauer KM, Schmidt SM, Ballestrero A, Grunebach F et al. Proteasome inhibitor bortezomib modulates TLR4-induced dendritic cell activation. Blood 2006; 108: 551–558.
Nencioni A, Garuti A, Schwarzenberg K, Cirmena G, Dal BG, Rocco I et al. Proteasome inhibitor-induced apoptosis in human monocyte-derived dendritic cells. Eur J Immunol 2006; 36: 681–689.
Adams J . The proteasome: a suitable antineoplastic target. Nat Rev Cancer 2004; 4: 349–360.
Hideshima T, Chauhan D, Richardson P, Anderson KC . Identification and validation of novel therapeutic targets for multiple myeloma. J Clin Oncol 2005; 23: 6345–6350.
Salceda S, Caro J . Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem 1997; 272: 22642–22647.
Birle DC, Hedley DW . Suppression of the hypoxia-inducible factor-1 response in cervical carcinoma xenografts by proteasome inhibitors. Cancer Res 2007; 67: 1735–1743.
Zhang J, Sattler M, Tonon G, Grabher C, Lababidi S, Zimmerhackl A et al. Targeting angiogenesis via a c-Myc/hypoxia-inducible factor-1alpha-dependent pathway in multiple myeloma. Cancer Res 2009; 69: 5082–5090.
Zhu K, Chan W, Heymach J, Wilkinson M, McConkey DJ . Control of HIF-1alpha expression by eIF2 alpha phosphorylation-mediated translational repression. Cancer Res 2009; 69: 1836–1843.
Hamner JB, Dickson PV, Sims TL, Zhou J, Spence Y, Ng CY et al. Bortezomib inhibits angiogenesis and reduces tumor burden in a murine model of neuroblastoma. Surgery 2007; 142: 185–191.
Moschetta M, Di PG, Ria R, Gnoni A, Mangialardi G, Guarini A et al. Bortezomib and zoledronic acid on angiogenic and vasculogenic activities of bone marrow macrophages in patients with multiple myeloma. Eur J Cancer 2010; 46: 420–429.
Basler M, Lauer C, Beck U, Groettrup M . The proteasome inhibitor bortezomib enhances the susceptibility to viral infection. J Immunol 2009; 183: 6145–6150.
Heider U, Rademacher J, Kaiser M, Kleeberg L, von MI, Sezer O . Decrease in CD4+ T-cell counts in patients with multiple myeloma treated with bortezomib. Clin Lymphoma Myeloma Leuk 2010; 10: 134–137.
Berges C, Haberstock H, Fuchs D, Sadeghi M, Opelz G, Daniel V et al. Proteasome inhibition activates the mitochondrial pathway of apoptosis in human CD4+ T cells. J Cell Biochem 2009; 108: 935–946.
Berges C, Haberstock H, Fuchs D, Miltz M, Sadeghi M, Opelz G et al. Proteasome inhibition suppresses essential immune functions of human CD4+ T cells. Immunology 2008; 124: 234–246.
Blanco B, Perez-Simon JA, Sanchez-Abarca LI, Caballero-Velazquez T, Gutierrez-Cossio S, Hernandez-Campo P et al. Treatment with bortezomib of human CD4+ T cells preserves natural regulatory T cells and allows the emergence of a distinct suppressor T-cell population. Haematologica 2009; 94: 975–983.
Meister S, Schubert U, Neubert K, Herrmann K, Burger R, Gramatzki M et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res 2007; 67: 1783–1792.
Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med 2008; 14: 748–755.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
All authors were involved in writing the manuscript.
Rights and permissions
About this article
Cite this article
Heine, A., Held, S., Bringmann, A. et al. Immunomodulatory effects of anti-angiogenic drugs. Leukemia 25, 899–905 (2011). https://doi.org/10.1038/leu.2011.24
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2011.24
Keywords
This article is cited by
-
Green nanotechnology of MGF-AuNPs for immunomodulatory intervention in prostate cancer therapy
Scientific Reports (2021)
-
Angiogenesis and immune checkpoint dual blockade in combination with radiotherapy for treatment of solid cancers: opportunities and challenges
Oncogenesis (2021)
-
Evolving Role of Immunotherapy in Metastatic Castration Refractory Prostate Cancer
Drugs (2021)
-
Combination Therapy in Renal Cell Carcinoma: the Best Choice for Every Patient?
Current Oncology Reports (2021)
-
Global survey of the immunomodulatory potential of common drugs
Nature Chemical Biology (2017)