Abstract
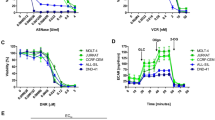
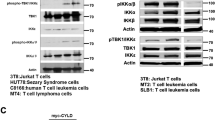
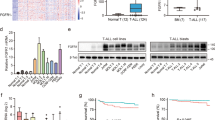
New treatments for adults with acute lymphoblastic T-cell leukemia (T-ALL) are urgently needed, as the current rate of overall remission in these patients is only about 40 percent. We recently showed the potential therapeutic benefit of the pegylated-human-arginase I (peg-Arg I) in T-ALL. However, the mechanisms by which peg-Arg I induces an anti-T-ALL effect remained unknown. Our results show the induction of T-ALL cell apoptosis by peg-Arg I, which associated with a global arrest in protein synthesis and with the phosphorylation of the eukaryotic-translation-initiation factor 2 alpha (eIF2α). Inhibition of eIF2α phosphorylation in T-ALL cells prevented the apoptosis induced by peg-Arg I, whereas the expression of a phosphomimetic eIF2α form increased the sensibility of T-ALL cells to peg-Arg I. Phosphorylation of eIF2α by peg-Arg I was mediated through kinases PERK and GCN2 and down-regulation of phosphatase GADD34. GCN2 and decreased GADD34 promoted T-ALL cell apoptosis after treatment with peg-Arg I, whereas PERK had an unexpected anti-apoptotic role. Additional results showed that phospho-eIF2α signaling further increased the anti-leukemic effects induced by peg-Arg I in T-ALL-bearing mice. These results suggest the central role of phospho-eIF2α in the anti-T-ALL effects induced by peg-Arg I and support its study as a therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Inaba H, Bhojwani D, Pauley JL, Pei D, Cheng C, Metzger ML et al. Combination chemotherapy with clofarabine, cyclophosphamide, and etoposide in children with refractory or relapsed haematological malignancies. Br J Haematol 2012; 156: 275–279.
Pui CH, Sandlund JT, Pei D, Campana D, Rivera GK, Ribeiro RC et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children’s Research Hospital. Blood 2004; 104: 2690–2696.
Tanosaki R, Tobinai K . Adult T-cell leukemia-lymphoma: current treatment strategies and novel immunological approaches. Expert Rev Hematol 2010; 3: 743–753.
Laport GF, Larson RA . Treatment of adult acute lymphoblastic leukemia. Semin Oncol 1997; 24: 70–82.
Hernandez CP, Morrow K, Lopez-Barcons LA, Zabaleta J, Sierra R, Velasco C et al. Pegylated arginase I: a potential therapeutic approach in T-ALL. Blood 2010; 115: 5214–5221.
Holcik M, Sonenberg N . Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 2005; 6: 318–327.
Woo CW, Cui D, Arellano J, Dorweiler B, Harding H, Fitzgerald KA et al. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol 2009; 11: 1473–1480.
Blander JM, Amsen D . Immunology. amino acid addiction. Science 2009; 324: 1282–1283.
Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, Harding HP et al. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J Cell Biol 2003; 163: 767–775.
Novoa I, Zeng H, Harding HP, Ron D . Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol 2001; 153: 1011–1022.
Chan SM, Weng AP, Tibshirani R, Aster JC, Utz PJ . Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia. Blood 2007; 110: 278–286.
Weng AP, Ferrando AA, Lee W, Morris JP, Silverman LB, Sanchez-Irizarry C et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 2004; 306: 269–271.
Sood R, Porter AC, Olsen DA, Cavener DR, Wek RC . A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2alpha. Genetics 2000; 154: 787–801.
Cheng PN, Lam TL, Lam WM, Tsui SM, Cheng AW, Lo WH et al. Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res 2007; 67: 309–317.
Muaddi H, Majumder M, Peidis P, Papadakis AI, Holcik M, Scheuner D et al. Phosphorylation of eIF2alpha at serine 51 is an important determinant of cell survival and adaptation to glucose deficiency. Mol Biol Cell 2010; 21: 3220–3231.
Rodriguez PC, Quiceno DG, Ochoa AC . L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 2007; 109: 1568–1573.
Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res 2009; 69: 1553–1560.
Jousse C, Averous J, Bruhat A, Carraro V, Mordier S, Fafournoux P . Amino acids as regulators of gene expression: molecular mechanisms. Biochem Biophys Res Commun 2004; 313: 447–452.
Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 2005; 307: 935–939.
Izzo F, Marra P, Beneduce G, Castello G, Vallone P, De R V et al. Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol 2004; 22: 1815–1822.
Ascierto PA, Scala S, Castello G, Daponte A, Simeone E, Ottaiano A et al. Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol 2005; 23: 7660–7668.
Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood 2010; 116: 5738–5747.
Hellen CU, Sarnow P . Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev 2001; 15: 1593–1612.
Schneider R, Agol VI, Andino R, Bayard F, Cavener DR, Chappell SA et al. New ways of initiating translation in eukaryotes. Mol Cell Biol 2001; 21: 8238–8246.
Talvas J, Obled A, Fafournoux P, Mordier S . Regulation of protein synthesis by leucine starvation involves distinct mechanisms in mouse C2C12 myoblasts and myotubes. J Nutr 2006; 136: 1466–1471.
Schewe DM, guirre-Ghiso JA . Inhibition of eIF2alpha dephosphorylation maximizes bortezomib efficiency and eliminates quiescent multiple myeloma cells surviving proteasome inhibitor therapy. Cancer Res 2009; 69: 1545–1552.
Acknowledgements
We thank Daniel Nguyen, BS for his technical assistance in some of the experiments. This work was supported in part by NIH-NCRR (COBRE) grants P20RR021970 and 1R21CA162133 to PCR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Morrow, K., Hernandez, C., Raber, P. et al. Anti-leukemic mechanisms of pegylated arginase I in acute lymphoblastic T-cell leukemia. Leukemia 27, 569–577 (2013). https://doi.org/10.1038/leu.2012.247
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.247
Keywords
This article is cited by
-
Arginine-dependent immune responses
Cellular and Molecular Life Sciences (2021)
-
Mechanisms of cell death induced by arginase and asparaginase in precursor B-cell lymphoblasts
Apoptosis (2019)
-
ER stress-induced mediator C/EBP homologous protein thwarts effector T cell activity in tumors through T-bet repression
Nature Communications (2019)
-
Arginase 1 promotes retinal neurovascular protection from ischemia through suppression of macrophage inflammatory responses
Cell Death & Disease (2018)
-
Sensitivity of Colorectal Cancer to Arginine Deprivation Therapy is Shaped by Differential Expression of Urea Cycle Enzymes
Scientific Reports (2018)