Abstract
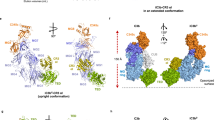
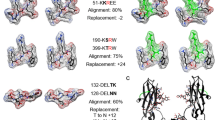
Pentraxins are a family of ancient innate immune mediators conserved throughout evolution. The classical pentraxins include serum amyloid P component (SAP) and C-reactive protein, which are two of the acute-phase proteins synthesized in response to infection1,2. Both recognize microbial pathogens and activate the classical complement pathway through C1q (refs 3 and 4). More recently, members of the pentraxin family were found to interact with cell-surface Fcγ receptors (FcγR) and activate leukocyte-mediated phagocytosis5,6,7,8. Here we describe the structural mechanism for pentraxin’s binding to FcγR and its functional activation of FcγR-mediated phagocytosis and cytokine secretion. The complex structure between human SAP and FcγRIIa reveals a diagonally bound receptor on each SAP pentamer with both D1 and D2 domains of the receptor contacting the ridge helices from two SAP subunits. The 1:1 stoichiometry between SAP and FcγRIIa infers the requirement for multivalent pathogen binding for receptor aggregation. Mutational and binding studies show that pentraxins are diverse in their binding specificity for FcγR isoforms but conserved in their recognition structure. The shared binding site for SAP and IgG results in competition for FcγR binding and the inhibition of immune-complex-mediated phagocytosis by soluble pentraxins. These results establish antibody-like functions for pentraxins in the FcγR pathway, suggest an evolutionary overlap between the innate and adaptive immune systems, and have new therapeutic implications for autoimmune diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Steel, D. M. & Whitehead, A. S. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol. Today 15, 81–88 (1994)
Pepys, M. B. et al. Comparative clinical study of protein SAP (amyloid P component) and C-reactive protein in serum. Clin. Exp. Immunol. 32, 119–124 (1978)
Garlanda, C., Bottazzi, B., Bastone, A. & Mantovani, A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu. Rev. Immunol. 23, 337–366 (2005)
Kaplan, M. H. & Volanakis, J. E. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J. Immunol. 112, 2135–2147 (1974)
Bharadwaj, D., Stein, M. P., Volzer, M., Mold, C. & Du Clos, T. W. The major receptor for C-reactive protein on leukocytes is Fcγ receptor II. J. Exp. Med. 190, 585–590 (1999)
Marnell, L. L., Mold, C., Volzer, M. A., Burlingame, R. W. & Du Clos, T. W. C-reactive protein binds to FcγRI in transfected COS cells. J. Immunol. 155, 2185–2193 (1995)
Bodman-Smith, K. B. et al. C-reactive protein-mediated phagocytosis and phospholipase D signalling through the high-affinity receptor for immunoglobulin G (FcγRI). Immunology 107, 252–260 (2002)
Bharadwaj, D., Mold, C., Markham, E. & Du Clos, T. W. Serum amyloid P component binds to Fcγ receptors and opsonizes particles for phagocytosis. J. Immunol. 166, 6735–6741 (2001)
Alles, V. V. et al. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood 84, 3483–3493 (1994)
Han, B. et al. TNFα-induced long pentraxin PTX3 expression in human lung epithelial cells via JNK. J. Immunol. 175, 8303–8311 (2005)
Ravetch, J. V. & Bolland, S. IgG Fc receptors. Annu. Rev. Immunol. 19, 275–290 (2001)
Bickerstaff, M. C. et al. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nature Med. 5, 694–697 (1999)
Xia, D. & Samols, D. Transgenic mice expressing rabbit C-reactive protein are resistant to endotoxemia. Proc. Natl Acad. Sci. USA 94, 2575–2580 (1997)
Mold, C., Rodriguez, W., Rodic-Polic, B. & Du Clos, T. W. C-reactive protein mediates protection from lipopolysaccharide through interactions with FcγR. J. Immunol. 169, 7019–7025 (2002)
Emsley, J. et al. Structure of pentameric human serum amyloid P component. Nature 367, 338–345 (1994)
Maxwell, K. F. et al. Crystal structure of the human leukocyte Fc receptor, FcγRIIa. Nature Struct. Biol. 6, 437–442 (1999)
Lawrence, M. C. & Colman, P. M. Shape complementarity at protein/protein interfaces. J. Mol. Biol. 234, 946–950 (1993)
Boyington, J. C., Motyka, S. A., Schuck, P., Brooks, A. G. & Sun, P. D. Crystal structure of an NK cell immunoglobulin-like receptor in complex with its class I MHC ligand. Nature 405, 537–543 (2000)
Ysern, X., Li, H. & Mariuzza, R. A. Imperfect interfaces. Nature Struct. Biol. 5, 412–414 (1998)
Hulett, M. D. & Hogarth, P. M. Molecular basis of Fc receptor function. Adv. Immunol. 57, 1–127 (1994)
Shrive, A. K. et al. Three dimensional structure of human C-reactive protein. Nature Struct. Biol. 3, 346–354 (1996)
Thompson, D., Pepys, M. B. & Wood, S. P. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure 7, 169–177 (1999)
Bang, R. et al. Analysis of binding sites in human C-reactive protein for FcγRI, FcγRIIA, and C1q by site-directed mutagenesis. J. Biol. Chem. 280, 25095–25102 (2005)
Tax, W. J., Willems, H. W., Reekers, P. P., Capel, P. J. & Koene, R. A. Polymorphism in mitogenic effect of IgG1 monoclonal antibodies against T3 antigen on human T cells. Nature 304, 445–447 (1983)
Clark, M. R., Stuart, S. G., Kimberly, R. P., Ory, P. A. & Goldstein, I. M. A single amino acid distinguishes the high-responder from the low-responder form of Fc receptor II on human monocytes. Eur. J. Immunol. 21, 1911–1916 (1991)
Parren, P. W. et al. On the interaction of IgG subclasses with the low affinity FcγRIIa (CD32) on human monocytes, neutrophils, and platelets. Analysis of a functional polymorphism to human IgG2. J. Clin. Invest. 90, 1537–1546 (1992)
Stein, M. P. et al. C-reactive protein binding to FcγRIIa on human monocytes and neutrophils is allele-specific. J. Clin. Invest. 105, 369–376 (2000)
Radaev, S., Motyka, S., Fridman, W. H., Sautes-Fridman, C. & Sun, P. D. The structure of a human type III Fcγ receptor in complex with Fc. J. Biol. Chem. 276, 16469–16477 (2001)
Mold, C., Baca, R. & Du Clos, T. W. Serum amyloid P component and C-reactive protein opsonize apoptotic cells for phagocytosis through Fcg receptors. J. Autoimmun. 19, 147–154 (2002)
Mold, C. & Du Clos, T. W. C-reactive protein increases cytokine responses to Streptococcus pneumoniae through interactions with Fcγ receptors. J. Immunol. 176, 7598–7604 (2006)
Sondermann, P. & Jacob, U. Human Fcγ receptor IIb expressed in Escherichia coli reveals IgG binding capability. Biol. Chem. 380, 717–721 (1999)
Du Clos, T. W. C-reactive protein reacts with the U1 small nuclear ribonucleoprotein. J. Immunol. 143, 2553–2559 (1989)
Potempa, L. A., Siegel, J. N., Fiedel, B. A., Potempa, R. T. & Gewurz, H. Expression, detection and assay of a neoantigen (Neo-CRP) associated with a free, human C-reactive protein subunit. Mol. Immunol. 24, 531–541 (1987)
Otwinowski, Z. M. W. processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Read, R. J. Pushing the boundaries of molecular replacement with maximum likelihood. Acta Crystallogr. D 57, 1373–1382 (2001)
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D 58, 1948–1954 (2002)
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
Brunger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Snyder, G. A., Brooks, A. G. & Sun, P. D. Crystal structure of the HLA-Cw3 allotype-specific killer cell inhibitory receptor KIR2DL2. Proc. Natl Acad. Sci. USA 96, 3864–3869 (1999)
Mustard, D. & Ritchie, D. W. Docking essential dynamics eigenstructures. Proteins 60, 269–274 (2005)
Delano, W. L. The PyMOL Molecular Graphics System <http://www.pymol.org> (2002)
Acknowledgements
We thank D. Klinman, G. Cheng, P. W. Dempsey and S. Bolland for providing the bone marrow from the Myd88-/-, RIP2-/- and wild-type C57BL/6 mice, respectively; M. Pancera and B. Dey for technical support in the isothermal titration calorimetry experiments; V. Deretic and S. Master for assistance with confocal microscopy; and B. Bottazzi for providing PTX-3. The X-ray SER-CAT beamlines (www.ser-cat.org/members.html) at the Advanced Photon Source is supported by the US Department of Energy, Basic Energy Sciences, Office of Science, under contract no. W-31-109-Eng-38. This work was supported by intramural research funding from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by RO1 AI28358 and by the Department of Veterans Affairs.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Notes, Supplementary Tables 1-3 and Supplementary Figures S1-S5 with Legends. (PDF 846 kb)
Rights and permissions
About this article
Cite this article
Lu, J., Marnell, L., Marjon, K. et al. Structural recognition and functional activation of FcγR by innate pentraxins. Nature 456, 989–992 (2008). https://doi.org/10.1038/nature07468
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07468
This article is cited by
-
Inactivation of pentraxin 3 suppresses M2-like macrophage activity and immunosuppression in colon cancer
Journal of Biomedical Science (2024)
-
Local Pentraxin-2 Deficit Is a Feature of Intestinal Fibrosis in Crohn’s Disease
Digestive Diseases and Sciences (2023)
-
Plasma proteome changes linked to late phase response after inhaled allergen challenge in asthmatics
Respiratory Research (2022)
-
Secretory quality control constrains functional selection-associated protein structure innovation
Communications Biology (2022)
-
Pentraxin 3 regulated by miR-224-5p modulates macrophage reprogramming and exacerbates osteoarthritis associated synovitis by targeting CD32
Cell Death & Disease (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.