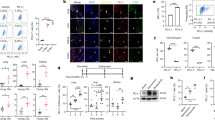
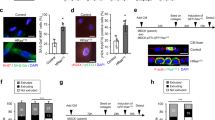
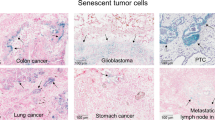
Abstract
Upon the aberrant activation of oncogenes, normal cells can enter the cellular senescence program, a state of stable cell-cycle arrest, which represents an important barrier against tumour development in vivo1. Senescent cells communicate with their environment by secreting various cytokines and growth factors, and it was reported that this ‘secretory phenotype’ can have pro- as well as anti-tumorigenic effects2,3,4,5. Here we show that oncogene-induced senescence occurs in otherwise normal murine hepatocytes in vivo. Pre-malignant senescent hepatocytes secrete chemo- and cytokines and are subject to immune-mediated clearance (designated as ‘senescence surveillance’), which depends on an intact CD4+ T-cell-mediated adaptive immune response. Impaired immune surveillance of pre-malignant senescent hepatocytes results in the development of murine hepatocellular carcinomas (HCCs), thus showing that senescence surveillance is important for tumour suppression in vivo. In accordance with these observations, ras-specific Th1 lymphocytes could be detected in mice, in which oncogene-induced senescence had been triggered by hepatic expression of NrasG12V . We also found that CD4+ T cells require monocytes/macrophages to execute the clearance of senescent hepatocytes. Our study indicates that senescence surveillance represents an important extrinsic component of the senescence anti-tumour barrier, and illustrates how the cellular senescence program is involved in tumour immune surveillance by mounting specific immune responses against antigens expressed in pre-malignant senescent cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Narita, M. & Lowe, S. W. Senescence comes of age. Nature Med. 11, 920–922 (2005)
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P. Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001)
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 455, 656–660 (2007)
Acosta, J. C. et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018 (2008)
Kuilman, T. et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 (2008)
Hickman, M. A. et al. Gene expression following direct injection of DNA into liver. Hum. Gene Ther. 5, 1477–1483 (1994)
Carlson, C. M., Frandsen, J. L., Kirchhof, N., McIvor, R. S. & Largaespada, D. A. Somatic integration of an oncogene-harboring Sleeping Beauty transposon models liver tumor development in the mouse. Proc. Natl Acad. Sci. USA 102, 17059–17064 (2005)
Khwaja, A., Rodriguez-Viciana, P., Wennstrom, S., Warne, P. H. & Downward, J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 16, 2783–2793 (1997)
Jackson, E. L. et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 15, 3243–3248 (2001)
Thomsen, M., Galvani, S., Canivet, C., Kamar, N. & Bohler, T. Reconstitution of immunodeficient SCID/beige mice with human cells: applications in preclinical studies. Toxicology 246, 18–23 (2008)
Tannour-Louet, M., Porteu, A., Vaulont, S., Kahn, A. & Vasseur-Cognet, M. A tamoxifen-inducible chimeric Cre recombinase specifically effective in the fetal and adult mouse liver. Hepatology 35, 1072–1081 (2002)
Harada, N. et al. Hepatocarcinogenesis in mice with β-catenin and Ha-ras gene mutations. Cancer Res. 64, 48–54 (2004)
Kamijo, T. et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91, 649–659 (1997)
Bettermann, K. et al. TAK1 suppresses a NEMO-dependent but NF-κB-independent pathway to liver cancer. Cancer Cell 17, 481–496 (2010)
Sarma, D. S., Rao, P. M. & Rajalakshmi, S. Liver tumour promotion by chemicals: models and mechanisms. Cancer Surv. 5, 781–798 (1986)
Brown, D. M. Cytolytic CD4 cells: direct mediators in infectious disease and malignancy. Cell. Immunol. 262, 89–95 (2010)
Frid, M. G. et al. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am. J. Pathol. 168, 659–669 (2006)
Silva, M. T. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J. Leukoc. Biol. 87, 93–106 (2010)
Hensel, M. et al. HIV and cancer in Germany. Dtsch. Arztebl. Int. 108, 117–122 (2011)
Sage, J., Miller, A. L., Perez-Mancera, P. A., Wysocki, J. M. & Jacks, T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature 424, 223–228 (2003)
Dirac, A. M. & Bernards, R. Reversal of senescence in mouse fibroblasts through lentiviral suppression of p53. J. Biol. Chem. 278, 11731–11734 (2003)
Acknowledgements
We thank D. Largaespada and M. Kay for providing transposon vectors and transposase encoding vectors. We thank L. Gröbe, A. Rinkel, N. Struever, H. Riedesel, the team of the Helmholtz Centre for Infection Research (HZI) animal facility, M. Rothe, K. Schulze, A. Kobold, F. Heinzmann, N. Jedicke, C. Schneider, M. Pesic, H. Klimek and A. Samuels for technical assistance and assistance with animal work, and F. Alves, S. Kimmina, C. Dullin and S. Greco for assistance with flat panel-volumetric computer tomography. We thank the tissue bank of the National Center for Tumor Diseases Heidelberg for providing liver explant tissues. We thank S. Lowe, H. Tillmann, F. Greten and members of the Lowe and Zender laboratory for advice and discussions. This work was supported by the Helmholtz Association of German Research Centres (VH-NG-424 to L.Z.), the German Research Foundation, DFG (Emmy Noether Programme ZE 545/2-1 to L.Z., SFB/TRR77 and the ‘Rebirth’ Cluster of Excellence), the Wilhelm Sander Stiftung, the Bear Necessities Pediatric Cancer Foundation, the Federal German Ministry for Education and Research (BMBF) (ARCHES AWARD to L.Z.) and the European Commission (project ‘Heptromic’). L.Z. holds an adjunct assistant Professorship with the Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, USA. This work is dedicated to J. Wehland.
Author information
Authors and Affiliations
Contributions
L.Z. designed and guided the research and wrote the manuscript. T.-W.K., T.Y. and N.W. conducted experiments and contributed to research design and manuscript preparation. L.H., T.W., D.D., A.H., M.G., R.R., A.P., M.I., M.V., S.W., M.H., S.K., J.G., F.T., To.L., D.B. M.M., M.O. and S.K. contributed to research design and/or conducted experiments. P.S. and Th.L. performed histopathological analyses.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
The file contains Supplementary Figures 1-18 with legends. (PDF 17342 kb)
Supplementary Methods
The file contains Supplementary Methods and additional references. (PDF 410 kb)
Rights and permissions
About this article
Cite this article
Kang, TW., Yevsa, T., Woller, N. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011). https://doi.org/10.1038/nature10599
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10599
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.