Abstract
Mutations or amplification of the MET proto-oncogene are involved in the pathogenesis of several tumours1,2,3,4, which rely on the constitutive engagement of this pathway for their growth and survival1,5. However, MET is expressed not only by cancer cells but also by tumour-associated stromal cells, although its precise role in this compartment is not well characterized6,7,8,9,10,11. Here we show that MET is required for neutrophil chemoattraction and cytotoxicity in response to its ligand hepatocyte growth factor (HGF). Met deletion in mouse neutrophils enhances tumour growth and metastasis. This phenotype correlates with reduced neutrophil infiltration to both the primary tumour and metastatic sites. Similarly, Met is necessary for neutrophil transudation during colitis, skin rash or peritonitis. Mechanistically, Met is induced by tumour-derived tumour necrosis factor (TNF)-α or other inflammatory stimuli in both mouse and human neutrophils. This induction is instrumental for neutrophil transmigration across an activated endothelium and for inducible nitric oxide synthase production upon HGF stimulation. Consequently, HGF/MET-dependent nitric oxide release by neutrophils promotes cancer cell killing, which abates tumour growth and metastasis. After systemic administration of a MET kinase inhibitor, we prove that the therapeutic benefit of MET targeting in cancer cells is partly countered by the pro-tumoural effect arising from MET blockade in neutrophils. Our work identifies an unprecedented role of MET in neutrophils, suggests a potential ‘Achilles’ heel’ of MET-targeted therapies in cancer, and supports the rationale for evaluating anti-MET drugs in certain inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gherardi, E., Birchmeier, W., Birchmeier, C. & Vande Woude, G. Targeting MET in cancer: rationale and progress. Nature Rev. Cancer 12, 89–103 (2012)
Bertotti, A. et al. Only a subset of Met-activated pathways are required to sustain oncogene addiction. Sci. Signal. 2, ra80 (2009)
Lennerz, J. K. et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J. Clin. Oncol. 29, 4803–4810 (2011)
Choueiri, T. K. et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J. Clin. Oncol. 31, 181–186 (2013)
Comoglio, P. M., Giordano, S. & Trusolino, L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nature Rev. Drug Discov. 7, 504–516 (2008)
Bussolino, F. et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 119, 629–641 (1992)
Liu, Y. et al. Hepatocyte growth factor and c-Met expression in pericytes: implications for atherosclerotic plaque development. J. Pathol. 212, 12–19 (2007)
Chen, Q., DeFrances, M. C. & Zarnegar, R. Induction of met proto-oncogene (hepatocyte growth factor receptor) expression during human monocyte-macrophage differentiation. Cell Growth Differ. 7, 821–832 (1996)
Baek, J. H., Birchmeier, C., Zenke, M. & Hieronymus, T. The HGF receptor/Met tyrosine kinase is a key regulator of dendritic cell migration in skin immunity. J. Immunol. 189, 1699–1707 (2012)
Adams, D. H. et al. Hepatocyte growth factor and macrophage inflammatory protein 1β: structurally distinct cytokines that induce rapid cytoskeletal changes and subset-preferential migration in T cells. Proc. Natl Acad. Sci. USA 91, 7144–7148 (1994)
Tesio, M. et al. Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood 117, 419–428 (2011)
Takeda, Y. et al. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature 479, 122–126 (2011)
Elliott, E. R. et al. Deletion of Syk in neutrophils prevents immune complex arthritis. J. Immunol. 187, 4319–4330 (2011)
Kishi, Y. et al. Systemic NK4 gene therapy inhibits tumor growth and metastasis of melanoma and lung carcinoma in syngeneic mouse tumor models. Cancer Sci. 100, 1351–1358 (2009)
Pennacchietti, S. et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 3, 347–361 (2003)
Moghul, A. et al. Modulation of c-MET proto-oncogene (HGF receptor) mRNA abundance by cytokines and hormones: evidence for rapid decay of the 8 kb c-MET transcript. Oncogene 9, 2045–2052 (1994)
Dai, J. Y., DeFrances, M. C., Zou, C., Johnson, C. J. & Zarnegar, R. The Met protooncogene is a transcriptional target of NFκB: implications for cell survival. J. Cell. Biochem. 107, 1222–1236 (2009)
Suga, H. et al. IFATS collection: fibroblast growth factor-2-induced hepatocyte growth factor secretion by adipose-derived stromal cells inhibits postinjury fibrogenesis through a c-Jun N-terminal kinase-dependent mechanism. Stem Cells 27, 238–249 (2009)
Fridlender, Z. G. & Albelda, S. M. Tumor-associated neutrophils: friend or foe? Carcinogenesis 33, 949–955 (2012)
Garber, K. MET inhibitors start on road to recovery. Nature Rev. Drug Discov. 13, 563–565 (2014)
Wright, H. L., Moots, R. J., Bucknall, R. C. & Edwards, S. W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 49, 1618–1631 (2010)
Passegué, E., Wagner, E. F. & Weissman, I. L. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell 119, 431–443 (2004)
Van Ziffle, J. A. & Lowell, C. A. Neutrophil-specific deletion of Syk kinase results in reduced host defense to bacterial infection. Blood 114, 4871–4882 (2009)
Abram, C. L., Roberge, G. L., Pao, L. I., Neel, B. G. & Lowell, C. A. Distinct roles for neutrophils and dendritic cells in inflammation and autoimmunity in motheaten mice. Immunity 38, 489–501 (2013)
Albanesi, M. et al. Neutrophils mediate antibody-induced antitumor effects in mice. Blood 122, 3160–3164 (2013)
Lagasse, E. & Clerc, R. G. Cloning and expression of two human genes encoding calcium-binding proteins that are regulated during myeloid differentiation. Mol. Cell. Biol. 8, 2402–2410 (1988)
Hamm, A. et al. PHD2 regulates arteriogenic macrophages through TIE2 signalling. EMBO Mol. Med. 5, 843–857 (2013)
Neufert, C., Becker, C. & Neurath, M. F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nature Protocols 2, 1998–2004 (2007)
Moolenbeek, C. & Ruitenberg, E. J. The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab. Anim. 15, 57–59 (1981)
Chen, X. & Calvisi, D. F. Hydrodynamic transfection for generation of novel mouse models for liver cancer research. Am. J. Pathol. 184, 912–923 (2014)
Schira, J. et al. Significant clinical, neuropathological and behavioural recovery from acute spinal cord trauma by transplantation of a well-defined somatic stem cell from human umbilical cord blood. Brain 135, 431–446 (2012)
Cramer, T. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 (2003)
Cotter, M. J., Norman, K. E., Hellewell, P. G. & Ridger, V. C. A novel method for isolation of neutrophils from murine blood using negative immunomagnetic separation. Am. J. Pathol. 159, 473–481 (2001)
Haslett, C., Guthrie, L. A., Kopaniak, M. M., Johnston, R. B., Jr & Henson, P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am. J. Pathol. 119, 101–110 (1985)
Shaked, Y. et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell 14, 263–273 (2008)
Acknowledgements
The authors thank: R. Stirparo, M. Mambretti and Y. Jönsson for technical assistance; G. Serini, L. Trusolino and P. Bruhns for comments; and E. Radaelli for valuable advice on histological analyses. V.F. and G.D.C. were supported by grants from the Fonds Wetenschappelijk Onderzoek (FWO), A.C. by the Fondazione Umberto Veronesi. S.W. is supported by a Wellcome Trust Senior Clinical Fellowship Award. M.M. is supported by a European Research Council starting grant.
Author information
Authors and Affiliations
Contributions
V.F. performed experimental design, all experiments, data acquisition and interpretation. G.D.C. performed in vitro assays and measured tumour experiments. M.D.M. performed ELISA assays, and designed and performed cloning strategies. J.S. performed all the bone marrow transplantations and in vivo tumour experiments. A.A.R.T. and S.W. performed neutrophil isolations and peritonitis assays. Z.G. provided the Mrp8 promoter. S.C. performed tumour experiments and skin rash assays in vivo. E.W. provided clinical samples. H.P. provided data interpretation on the CRC and HCC models. A.C. performed experimental design, mouse tumour experiments, analysis of histological stainings and FACS, data acquisition and interpretation. M.M. performed experimental design, data analysis, conducted scientific direction and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
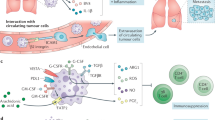
Extended Data Figure 1 Scheme illustrating the role of MET in neutrophils.
During cancer or infections, the release of cytokines such as IL-1 at the inflammatory site will promote the expression of TNF-α on the endothelium and the surrounding tissue. When circulating neutrophils encounter the activated endothelium, TNF-α will unleash NF-κB through binding to TNFR1, which in turn will induce MET expression on the neutrophil surface. HGF, also released and proteolytically activated at the site of inflammation, will bind to MET and stimulate the firm adhesion of neutrophils to the endothelium, probably via integrin engagement, and thus neutrophil diapedesis. Once extravasated, the HGF/MET pathway will still function on neutrophils by reinforcing their cytotoxic response through the induction of iNOS and NO production, ultimately favouring a bactericidal and tumoricidal neutrophil phenotype.
Extended Data Figure 2 Met deletion in immune cells, but not in endothelial cells, fosters tumour growth.
a, MET expression in total bone marrow (BM) cells, endothelial cells (EC) and neutrophils harvested from Met floxed mice intercrossed with the Tie2:Cre deleter thus generating Tie2;Metfl/fl (KO) or Tie2;Metwt/wt (WT) mice. Western blots are representative of three repetitions on independent biological replicates. Western blot images have been cropped for presentation. Full scan images are shown in Supplementary Fig. 1. b–d, Quantification (b) and representative images of tumour sections’ TdT-mediated dUTP nick end labelling (TUNEL) stainings (c, d) from subcutaneous end-stage LLC tumours in WT→WT and KO→WT mice. Data combine two independent experiments; total n = 10 mice per condition. e, FACS quantification of AnnexinV+ 7AAD− early apoptotic tumour cells in WT→WT and KO→WT mice. Data combine two independent experiments; total n = 8 mice per condition. f–j, Tumour necrosis quantification in WT→WT and KO→WT mice (f), assessed by histological evaluation of H&E-stained tumour sections (g, h) and by measurement of autofluorescent tumour areas (i, j); yellow dotted lines demarcate necrosis. Data combine two independent experiments; total n = 10 mice per condition. k–m, Quantification (k) and representative images of tumour sections stained for the proliferation marker pHH3 (l, m) from subcutaneous end-stage LLC tumours in WT→WT and KO→WT mice. Data combine two independent experiments; total n = 10 mice per condition. n, FACS quantification of BrdU+ proliferating tumour cells in WT→WT and KO→WT mice. Data combine two independent experiments; total n = 10 mice per condition. o–r, CD31+ vessel area (o), vessel density (p), lectin perfusion (q) and hypoxic (Pimo+) area (r) in LLC subcutaneous tumours from KO→WT mice (in which the haematopoietic/immune system is knocked out for Met) or WT→KO mice (in which endothelial cells only are knocked out for Met) compared to control WT→WT mice. Data in o–r combine two independent experiments; total n: WT→WT, 12; KO→WT, 8; WT→KO, 8. s–u, Subcutaneous LLC tumour growth (s), weight (t) and lung metastases (u) in Tie2;Metfl/fl compared to Tie2;Metwt/wt mice. Data combine two independent experiments; total n: Tie2;Metwt/wt , 12; Tie2;Metfl/fl , 10. v, Subcutaneous LLC tumour growth in endothelial-cell-specific Met KO (WT→KO) and control (WT→WT) mice. Data combine two independent experiments; total n = 8 per condition. *P < 0.05 versus WT→WT (b, e, f, k, n), versus Tie2;Metwt/wt (s–u). Scale bars: 50 μm (c, d, l, m); 100 μm (g–j). All graphs show mean ± s.e.m.
Extended Data Figure 3 Circulating and tumour-infiltrating immune cells upon Met deletion.
a–e, FACS analysis showing percentages of circulating monocytes (a), lymphocytes (b), neutrophils (c), eosinophils (d) and basophils (e) in tumour-free or in LLC-tumour-bearing WT→WT and KO→WT mice. Data combine two independent experiments; total n = 8 mice per condition. f, Quantification of LLC tumour sections stained for the pan-leukocyte marker CD45, the macrophage marker F4/80, the NK marker NK1.1, the B lymphocyte marker CD45R, the T helper cell marker CD4, the cytotoxic T cell marker CD8 and the dendritic cell marker CD11c (with exclusion of F4/80+ area) in WT→WT and KO→WT mice. Data combine two independent experiments; total n = 8 mice per condition). g, h, FACS quantification for tumour-associated CD45+ leukocytes (g) or CD45+ IgE+ CD49b+ CD4− CD45R− basophils and CD45+ CD11b+ SiglecF+ Ly6Cmed F4/80low MHCII− eosinophils (h) in WT→WT and KO→WT mice. Data combine two independent experiments; total n = 8 mice per condition. i, j, FACS quantification (i) and gating strategy (j) for tumour-associated neutrophils selected from the main tumour cell population negative for 7AAD staining; tumour-associated neutrophils were then gated as CD11b and Ly6G double-positive cells. Data combine two independent experiments; total n: WT→WT, 11; KO→WT, 10. k, Ly6G+ tumour infiltration at day 9, day 13 and day 19 after LLC subcutaneous tumour injection in WT→WT and KO→WT mice. Data combine two independent experiments; total n = 8 mice per condition. l, Morphometric quantification of leukocytes and macrophages on CD45- and F480-stained lung sections, respectively, from LLC-tumour-bearing WT→WT or KO→WT mice. Data combine two independent experiments; total n = 8 mice per condition. m, FACS quantification of CD11b+ Ly6G+ neutrophils and CD11b+ F4/80+ macrophages infiltrating metastatic lungs from LLC-tumour-bearing WT→WT or KO→WT mice. Data combine two independent experiments; total n = 8 mice per condition. *P < 0.05 versus WT→WT (f, g, i, k–m); †P < 0.05 versus tumour free (a–d). All graphs show mean ± s.e.m.
Extended Data Figure 4 MET in neutrophils is required for their anti-tumour activity.
a, b, Western blot analysis (a) and relative densitometric analysis (b) for MET expression in bone marrow neutrophils and monocytes upon reconstitution of WT recipient mice by WT or KO HSPCs transduced in vitro with an empty vector (Mrp8:Empty) or a vector expressing Met under the neutrophil-specific promoter Mrp8 (Mrp8:Met); tubulin was used as loading control. Western blots are representative of three repetitions on independent biological samples where each sample is the pool of neutrophils or monocytes isolated from three mice. Densitometric analysis was performed on these three western blots. A.U., arbitrary units. c, FACS analysis for green fluorescent protein (GFP) in circulating Ly6G+ neutrophils or CD115+ monocytes, harvested from the neutrophil-specific Mrp8:Cre line carrying separate expression of GFP because of an internal ribosome entry site (IRES) downstream the Mrp8-driven cre gene. Data combine two independent experiments; total n = 10 mice per condition. d, MET expression in neutrophils, monocytes and macrophages harvested from Mrp8;Metwt/wt or Mrp8;Metfl/fl mice. Western blots are representative of three repetitions on independent biological replicates. e, FACS analysis for CD11b+ Ly6G+ neutrophils in subcutaneous LLC tumours from Mrp8;Metwt/wt or Mrp8;Metfl/fl . Data combine two independent experiments; total n: Mrp8;Metwt/wt , 10; Mrp8;Metfl/fl , 11. Western blot images in a, d have been cropped for presentation. Full scan images are shown in Supplementary Fig. 1. *P < 0.05 versus Mrp8:Empty WT→WT (b), versus Mrp8;Metwt/wt (e); †P < 0.05 versus Mrp8:Empty WT→WT; ‡P < 0.05 versus Mrp8:Empty KO→WT. All graphs show mean ± s.e.m.
Extended Data Figure 5 Pharmacological and genetic inhibition of MET prevents the recruitment of anti-tumoural neutrophils to several neoplastic tissues and inflammatory sites.
a, Tumour weight of subcutaneous B16F10 melanomas in WT→WT and KO→WT mice. Data combine two independent experiments; total n: WT→WT, 8; KO→WT, 9. b, c, Total tumour weight (b) and metastatic index (c) in MMTV-PyMT mice reconstituted with WT or Met KO bone marrow cells before tumour appearance (WT→PyMT and KO→PyMT mice, respectively). Data combine three independent experiments; total n: WT→PyMT, 13; KO→PyMT, 16. d, e, FACS quantification for CD11b+ Ly6G+ neutrophils in T241 tumours harvested from WT→WT or KO→WT mice (d) or in in breast tumours spontaneously grown in WT→PyMT and KO→PyMT mice (e). Data combine two independent experiments; total n = 10 mice per condition (d) or total n = 8 mice per condition (e). f–i, Length measurement (f) and representative image (g) of the colon, as well as quantification of neutrophils (h) and macrophages (i) on bowel sections, from WT→WT and KO→WT mice upon induction of chronic colitis compared to healthy control. Data combine two independent experiments; total n: healthy, 5; WT→WT, 12; KO→WT, 15. j, k, Tumour weight (j) and metastatic mesenteric lymph nodes (k) 12 days after orthotopic injection of pancreatic Panc02 cancer cells in WT→WT and KO→WT mice. Data combine two independent experiments; total n = 12 per condition. l, Histological quantification of Ly6G+ infiltrates in Panc02 pancreatic tumours harvested from WT→WT and KO→WT mice. Data combine two independent experiments; total n = 12 mice per condition. m, Quantification of plasma HGF in tumour (TM)-free mice, in subcutaneous LLC or orthotopic Panc02 tumour-bearing mice. Data combine two independent experiments; total n: tumour free, 10; LLC, 10; Panc02, 8 biological replicates. n, Quantification of HGF in subcutaneous LLC or orthotopic Panc02 tumours. Data combine two independent experiments; total n: LLC, 10; Panc02, 8 biological replicates. o, p, Quantification of HGF in plasma (o) or in subcutaneous LLC tumours (p) from tumour-bearing WT→WT and KO→WT mice. Data are representative of two independent experiments using 5 mice per condition per experiment. q, Quantification of Ly6G+ area on sections from B16F10 melanomas grown in C57BL/6 WT mice, daily treated with PF-04217903, INCB28060, JNJ-38877605, or vehicle as control. Data combine two independent experiments; total n: vehicle, 14; PF-04217903, 9; INCB28060, 6; JNJ-38877605, 4. r, Western blot analysis for MET in B16F10 melanoma cells after transduction with a lentiviral vector encoding scrambled or mouse shMet under a constitutive promoter; vinculin was used as loading control. Western blot is representative of three independent repetitions. Western blot images have been cropped for presentation. Full scan images are shown in Supplementary Fig. 1. *P < 0.05 versus WT→WT (a, d, h), versus WT→PyMT (b, e), versus LLC (m, n), versus vehicle (q); †P < 0.05 versus healthy (f, h, i), versus tumour free (m). Scale bar: 10 mm (g). All graphs show mean ± s.e.m.
Extended Data Figure 6 HGF is required for MET activation upon induction by TNF-α.
a, Gating strategy related to Fig. 3b to quantify MET expression in blood neutrophils from LLC-tumour (TM)-bearing mice and in TANs, where live cells were first gated as CD11b-positive cells; this population was finally gated for Ly6G and MET to identify MET-expressing Ly6G+ neutrophils. b, c, qRT–PCR for MET in mouse (b) and human (c) neutrophils after LPS or TNF-α stimulation. Data are representative of three independent experiments using four biological replicates per condition per experiment. d, e, qRT–PCR for MET expression in mouse (d) or human (e) neutrophils cultured in normoxia (21% O2) or hypoxia (1% O2). Data combine two independent experiments; total n = 8 biological replicates per condition. f, g, ELISA for total MET (f) and phospho-MET (g) from mouse neutrophils stimulated for 3 min with mock medium or HGF after an overnight incubation with or without TNF-α. Data combine three independent experiments; total n = 6 biological replicates per condition. h, HGF release by neutrophils stimulated with mock medium or TNF-α after 20 h in culture. Data combine two independent experiments; total n = 6 biological replicates per condition. i, qRT–PCR for TNFA in HUVECs upon stimulation with IL-1α compared to mock medium. Data combine two independent experiments; total n = 4 biological replicates per condition. j, qRT–PCR for Met in mouse neutrophils co-cultured with HUVEC/NS or HUVEC/IL transduced with shTNFA or scramble as control. Data are representative of three independent experiments in which three different shRNA sequences were used; total n = 4 biological replicates per condition per experiment. k, l, qRT–PCR for Met in WT, TNFR1 KO or TNRF2 KO neutrophils upon co-culture with HUVEC/NS or HUVEC/IL (k), or after stimulation with conditioned medium (TCM) from LLC tumours (l). Data are representative of two independent experiments using four biological replicates per condition per experiment. m, qRT–PCR for MET in human neutrophils stimulated with A549-CCM in the presence or absence of Enbrel or human IgG as control. Data are representative of two independent experiments using four biological replicates per condition per experiment. *P < 0.05 versus mock (b, c, i), versus TNF-α alone (g), versus HUVEC/NS (j), versus WT (k, l), versus A549-CCM (m); †P < 0.05 versus untreated or HGF alone (f, g), versus HUVEC/NS (k), versus mock (l, m). Graph shows mean ± s.e.m.
Extended Data Figure 7 Met deletion in neutrophils does not affect apoptosis.
a, b, Gating strategy of apoptotic WT (a) and Met KO (b) neutrophils in LLC tumours where single-cell suspensions were first gated for physical parameters and then for CD11b and Ly6G to identify neutrophils as double-positive cells; this population was finally gated for AnnexinV and 7AAD: AnnexinV+ 7AAD− cells display early apoptotic neutrophils whereas AnnexinV+ 7AAD+ cells display late apoptotic neutrophils. c, Quantification of apoptotic WT and Met KO tumour-associated neutrophils measured by FACS. Data combine two independent experiments; total n = 7 mice per condition. d, Quantification of apoptotic WT and Met KO neutrophils on LLC tumour sections by immunohistochemistry. Data combine two independent experiments; total n: WT→WT, 7; KO→WT, 6. e, FACS analysis for AnnexinV and 7AAD of WT or KO neutrophils incubated for 10 h in the presence or absence of LPS and HGF, alone or in combination. Data combine two independent experiments; total n = 6 biological replicates per condition. †P < 0.05 versus untreated or HGF alone. Graph shows mean ± s.e.m.
Extended Data Figure 8 MET affects neither neutrophil basal migration nor polarization but it is required for neutrophil recruitment and cytotoxicity.
a, Quantification of Ly6G staining in ear sections upon phorbol ester (TPA)-induced cutaneous rash in Mrp8;Metwt/wt and Mrp8;Metfl/fl mice. Data combine two independent experiments; total n = 8 mice per condition. b, FACS analysis on peritoneal lavages for Ly6G+ neutrophils or F4/80+ macrophages in Mrp8;Metwt/wt and Mrp8;Metfl/fl mice 4 h after intraperitoneal injection of sterile zymosan A. Data are representative of two independent experiments using 5 mice per condition per experiment. c, d, Quantification of F4/80 (c) and CD3 (d) stainings in ear sections at baseline and upon TPA-induced cutaneous rash. Data combine two independent experiments; total n: WT→WT control (CTRL), 22; KO→WT CTRL, 15; WT→WT TPA, 23; KO→WT TPA, 15 (c); or total n = 8 mice per condition (d). e, FACS quantification of Mrp8;Metwt/wt and Mrp8;Metfl/fl neutrophils recruited into subcutaneous air pouches in response to HGF, CXCL1 or PBS. Data combine two independent experiments; total n = 6 mice per condition. f, FACS quantification of WT neutrophil adhesion to quiescent HUVECs (HUVEC/NS) or activated HUVECs (HUVEC/IL) in the presence or absence of HGF. Data are representative of two independent experiments using four biological replicates per condition per experiment. g, h, FACS quantification of WT and Met KO neutrophils migrated through a bare porous filter (that is, in the absence of HUVECs) towards HGF (g) or tumour conditioned medium (TCM) (h). Data are representative of two independent experiments using three biological replicates per condition per experiment. i, Gene expression profile for N1 and N2 markers in neutrophils sorted from LLC tumours grown in WT→WT or KO→WT mice. Data are representative of three independent experiments using 4 mice per condition per experiment. j, Cytotoxicity of WT and KO tumour-associated neutrophils against T241 cells in the absence or presence of the NO synthase inhibitor L-NMMA. Data are representative of three independent experiments using three biological replicates per condition per experiment. k, FACS quantification of DAF-FM-positive circulating neutrophils after co-culture with LLC cancer cells as a readout of NO production in the absence or presence of HGF. Data are representative of four independent experiments using three biological replicates per condition per experiment. l, Quantification of LLC cancer cell killing by WT and KO neutrophils (isolated from the blood of tumour-bearing mice), stimulated with HGF alone or in the presence of L-NMMA. Data are representative of two independent experiments using n = 12 biological replicates per condition per experiment. m, Blood neutrophils in WT→WT and KO→WT mice treated with neutrophil-depleting Ly6G antibody or rat IgG as control. Data combine two independent experiments; total n = 16 per condition. *P < 0.05 versus Mrp8;Metwt/wt (a, b), versus Mrp8;Metwt/wt + HGF (e), versus HUVEC/NS (f), versus WT→WT untreated (j), versus WT→WT + HGF (k,l); †P < 0.05 versus CTRL (c, d), versus PBS (e), versus mock (f, h), versus WT→WT untreated (j–l), versus IgG (m); ‡P < 0.05 versus WT→WT + HGF (l). All graphs show mean ± s.e.m.
Supplementary information
Supplementary Information
This file contains Supplementary Figure 1, which shows the Western blot scans for Figure 3 and Extended Data Figures 2, 4 and 5, and Supplementary Tables 1 and 2. (PDF 415 kb)
Rights and permissions
About this article
Cite this article
Finisguerra, V., Di Conza, G., Di Matteo, M. et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature 522, 349–353 (2015). https://doi.org/10.1038/nature14407
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14407
This article is cited by
-
Dormancy of cutaneous melanoma
Cancer Cell International (2024)
-
Neutrophils in cancer: dual roles through intercellular interactions
Oncogene (2024)
-
The role of stromal cells in epithelial–mesenchymal plasticity and its therapeutic potential
Discover Oncology (2024)
-
Bioinformatics analyses of combined databases identify shared differentially expressed genes in cancer and autoimmune disease
Journal of Translational Medicine (2023)
-
Exploiting innate immunity for cancer immunotherapy
Molecular Cancer (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.