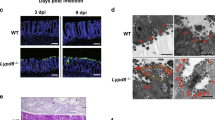
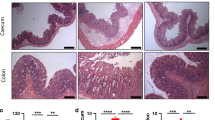
Abstract
Suppression of inflammation is critical for effective therapy of many infectious diseases. However, the high rates of mortality caused by sepsis attest to the need to better understand the basis of the inflammatory sequelae of sepsis and to develop new options for its treatment. In mice, inflammatory responses to host danger-associated molecular patterns (DAMPs), but not to microbial pathogen-associated molecular patterns (PAMPs), are repressed by the interaction of CD24 and SiglecG (SIGLEC10 in human). Here we use an intestinal perforation model of sepsis to show that microbial sialidases target the sialic acid–based recognition of CD24 by SiglecG/10 to exacerbate inflammation. Sialidase inhibitors protect mice against sepsis by a mechanism involving both CD24 and Siglecg, whereas mutation of either gene exacerbates sepsis. Analysis of sialidase-deficient bacterial mutants confirms the key contribution of disrupting sialic acid–based pattern recognition to microbial virulence and supports the clinical potential of sialidase inhibition for dampening inflammation caused by infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
18 January 2012
In the version of this article initially published, a line in the abstract read, “repressed by the t interaction….” It should have read, “repressed by the interaction….” The error has been corrected in the HTML and PDF versions of the article.
References
Favre, D. et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 5, e1000295 (2009).
Dombrovskiy, V.Y., Martin, A.A., Sunderram, J. & Paz, H.L. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit. Care Med. 35, 1244–1250 (2007).
Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J.M. & Hoffmann, J.A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983 (1996).
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C.A. Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997).
Inohara, N., Ogura, Y. & Nunez, G. Nods: a family of cytosolic proteins that regulate the host response to pathogens. Curr. Opin. Microbiol. 5, 76–80 (2002).
Ting, J.P., Willingham, S.B. & Bergstralh, D.T. NLRs at the intersection of cell death and immunity. Nat. Rev. Immunol. 8, 372–379 (2008).
Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 (1994).
Kono, H. & Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 8, 279–289 (2008).
Wang, H. et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 (1999).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050–1059 (2007).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008).
Chen, G.Y., Tang, J., Zheng, P. & Liu, Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 323, 1722–1725 (2009).
Crocker, P.R., Paulson, J.C. & Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7, 255–266 (2007).
Varki, A. Multiple changes in sialic acid biology during human evolution. Glycoconj. J. 26, 231–245 (2009).
Ding, C. et al. Siglecg limits the size of B1a B cell lineage by down-regulating NFκB activation. PLoS ONE 2, e997 (2007).
Hoffmann, A. et al. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat. Immunol. 8, 695–704 (2007).
Duong, B.H. et al. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J. Exp. Med. 207, 173–187 (2010).
Carlin, A.F. et al. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 113, 3333–3336 (2009).
Jedrzejas, M.J. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 65, 187–207 (2001).
Mally, M., Shin, H., Paroz, C., Landmann, R. & Cornelis, G.R. Capnocytophaga canimorsus: a human pathogen feeding at the surface of epithelial cells and phagocytes. PLoS Pathog. 4, e1000164 (2008).
Peltola, V.T., Murti, K.G. & McCullers, J.A. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J. Infect. Dis. 192, 249–257 (2005).
Tong, H.H., Blue, L.E., James, M.A. & DeMaria, T.F. Evaluation of the virulence of a Streptococcus pneumoniae neuraminidase-deficient mutant in nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect. Immun. 68, 921–924 (2000).
Rittirsch, D., Huber-Lang, M.S., Flierl, M.A. & Ward, P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36 (2009).
Brocker, T., Riedinger, M. & Karjalainen, K. Driving gene expression specifically in dendritic cells. Adv. Exp. Med. Biol. 417, 55–57 (1997).
Chen, M. et al. Dendritic cell apoptosis in the maintenance of immune tolerance. Science 311, 1160–1164 (2006).
Blixt, O., Collins, B.E., van den Nieuwenhof, I.M., Crocker, P.R. & Paulson, J.C. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J. Biol. Chem. 278, 31007–31019 (2003).
Li, Y. et al. Identifying selective inhibitors against the human cytosolic sialidase NEU2 by substrate specificity studies. Mol. Biosyst. 7, 1060–1072 (2011).
Lee, L.T. & Howe, C. Pneumococcal neuraminidase. J. Bacteriol. 91, 1418–1426 (1966).
O'Brien, K.L. et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374, 893–902 (2009).
Liu, Y., Chen, G.Y. & Zheng, P. CD24-Siglec G/10 discriminates danger- from pathogen-associated molecular patterns. Trends Immunol. 30, 557–561 (2009).
Liu, Y., Chen, G.Y. & Zheng, P. Sialoside-based pattern recognitions discriminating infections from tissue injuries. Curr. Opin. Immunol. (2010).
Piagnerelli, M. et al. Neuraminidase alters red blood cells in sepsis. Crit. Care Med. 37, 1244–1250 (2009).
Grewal, P.K. et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat. Med. 14, 648–655 (2008).
Drzeniek, R. Viral and bacterial neuraminidases. Curr. Top. Microbiol. Immunol. 59, 35–74 (1972).
Jedrzejas, M.J. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 65, 187–207 (2001).
Cavassani, K.A. et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J. Exp. Med. 205, 2609–2621 (2008).
Janeway, C. Immunogenicity signals 1,2,3...and 0. Immunol. Today [news] 10, 283–286 (1989).
Rittirsch, D., Flierl, M.A. & Ward, P.A. Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 8, 776–787 (2008).
Rittirsch, D., Hoesel, L.M. & Ward, P.A. The disconnect between animal models of sepsis and human sepsis. J. Leukoc. Biol. 81, 137–143 (2007).
Yu, H. et al. A multifunctional Pasteurella multocida sialyltransferase: a powerful tool for the synthesis of sialoside libraries. J. Am. Chem. Soc. 127, 17618–17619 (2005).
Yu, H. et al. Highly efficient chemoenzymatic synthesis of naturally occurring and non-natural alpha-2,6-linked sialosides: a P. damsela alpha-2,6-sialyltransferase with extremely flexible donor-substrate specificity. Angew. Chem. Int. Edn Engl. 45, 3938–3944 (2006).
Li, Y. et al. Pasteurella multocida sialic acid aldolase: a promising biocatalyst. Appl. Microbiol. Biotechnol. 79, 963–970 (2008).
Nielsen, P.J. et al. Altered erythrocytes and a leaky block in B-cell development in CD24/HSA-deficient mice. Blood 89, 1058–1067 (1997).
Tettelin, H. et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293, 498–506 (2001).
King, S.J. et al. Phase variable desialylation of host proteins that bind to Streptococcus pneumoniae in vivo and protect the airway. Mol. Microbiol. 54, 159–171 (2004).
Burnaugh, A.M., Frantz, L.J. & King, S.J. Growth of Streptococcus pneumoniae on human glycoconjugates is dependent upon the sequential activity of bacterial exoglycosidases. J. Bacteriol. 190, 221–230 (2008).
Lutz, M.B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999).
Acknowledgements
We thank P.N. Boyaka for valuable advice, Y. Chen for critical reading of the manuscript and valuable discussions and D. Kroft for editorial assistance. This study is supported by grants from US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
G.-Y.C., K.A.C., J.C., H.C., H.Y., X.Z., D.F., W.W., J.Q., S.A.W., C.H., C.C. and L.S. performed experiments, C.M.H., and S.L.K. advised on CLP model, X.-F. B. and J.-Q.L. provided transgenic mice, Y.L., P.Z. and G.-Y.C. designed the overall study. X.C. supervised synthesis of sialosides and sialo-modifications of CD24Fc, while S.K. supervised production of mutant bacteria. Y.L., P.Z. and G.-Y.C. wrote the manuscript with input from other authors.
Corresponding authors
Ethics declarations
Competing interests
Y.L., P.Z., G.-Y.C., X.C. and S.K. are co-inventors of a pending patent application that has been licensed to OncoImmune, Inc., of which Y.L. and P.Z. are among the co-founders and have equity interest.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1 and Supplementary Figs. 1–5 (PDF 476 kb)
Rights and permissions
About this article
Cite this article
Chen, GY., Chen, X., King, S. et al. Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat Biotechnol 29, 428–435 (2011). https://doi.org/10.1038/nbt.1846
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1846
This article is cited by
-
Emerging phagocytosis checkpoints in cancer immunotherapy
Signal Transduction and Targeted Therapy (2023)
-
Ablation of Siglec-E augments brain inflammation and ischemic injury
Journal of Neuroinflammation (2022)
-
The new progress in cancer immunotherapy
Clinical and Experimental Medicine (2022)
-
Knock-Down of CD24 in Astrocytes Aggravates Oxyhemoglobin-Induced Hippocampal Neuron Impairment
Neurochemical Research (2022)
-
Extracellular CIRP decreases Siglec-G expression on B-1a cells skewing them towards a pro-inflammatory phenotype in sepsis
Molecular Medicine (2021)