Abstract
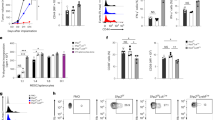
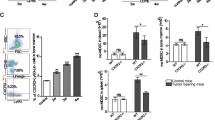
Two major populations of myeloid-derived suppressor cells (MDSCs), monocytic MDSCs (M-MDSCs) and polymorphonuclear MDSCs (PMN-MDSCs) regulate immune responses in cancer and other pathologic conditions. Under physiologic conditions, Ly6ChiLy6G− inflammatory monocytes, which are the normal counterpart of M-MDSCs, differentiate into macrophages and dendritic cells. PMN-MDSCs are the predominant group of MDSCs that accumulates in cancer. Here we show that a large proportion of M-MDSCs in tumor-bearing mice acquired phenotypic, morphological and functional features of PMN-MDSCs. Acquisition of this phenotype, but not the functional attributes of PMN-MDSCs, was mediated by transcriptional silencing of the retinoblastoma gene through epigenetic modifications mediated by histone deacetylase 2 (HDAC-2). These data demonstrate a new regulatory mechanism of myeloid cells in cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Gabrilovich, D.I. et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 67, 425 (2007).
Gabrilovich, D.I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Gabrilovich, D.I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Ostrand-Rosenberg, S. & Sinha, P. Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 182, 4499–4506 (2009).
Cuenca, A.G. et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol. Med. 17, 281–292 (2011).
Highfill, S.L. et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood 116, 5738–5747 (2010).
Jeisy-Scott, V. et al. Increased MDSC accumulation and Th2 biased response to influenza A virus infection in the absence of TLR7 in mice. PLoS ONE 6, e25242 (2011).
Chen, S., Akbar, S.M., Abe, M., Hiasa, Y. & Onji, M. Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clin. Exp. Immunol. 166, 134–142 (2011).
Zhu, X., Herrera, G. & Ochoa, J.B. Immunosupression and infection after major surgery: a nutritional deficiency. Crit. Care Clin. 26, 491–500 (2010).
Sander, L.E. et al. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J. Exp. Med. 207, 1453–1464 (2010).
Youn, J.I., Nagaraj, S., Collazo, M. & Gabrilovich, D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 181, 5791–5802 (2008).
Movahedi, K. et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T-cell suppressive activity. Blood 111, 4233–4244 (2008).
Dolcetti, L. et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur. J. Immunol. 40, 22–35 (2010).
Peranzoni, E. et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr. Opin. Immunol. 22, 238–244 (2010).
Auffray, C., Sieweke, M.H. & Geissmann, F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 27, 669–692 (2009).
Youn, J.-I., Collazo, M., Shalova, I., Biswas, S. & Gabrilovich, D. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J. Leukoc. Biol. 91, 167–181 (2012).
Brandau, S. et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J. Leukoc. Biol. 89, 311–317 (2011).
Nagaraj, S. & Gabrilovich, D.I. Myeloid-derived suppressor cells in human cancer. Cancer J. 16, 348–353 (2010).
Sonda, N., Chioda, M., Zilio, S., Simonato, F. & Bronte, V. Transcription factors in myeloid-derived suppressor cell generation. Curr. Opin. Immunol. 23, 279–285 (2011).
Condamine, T. & Gabrilovich, D.I. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 32, 19–25 (2011).
Nagaraj, S. et al. Anti-inflammatory triterpenoid blocks immune suppressive function of myeloid-derived suppressor cells and improves immune response in cancer. Clin. Cancer Res. 16, 1812–1823 (2010).
Solito, S. et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood 118, 2254–2265 (2011).
Zea, A.H. et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 65, 3044–3048 (2005).
Filipazzi, P. et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J. Clin. Oncol. 25, 2546–2553 (2007).
Poschke, I., Mougiakakos, D., Hansson, J., Masucci, G.V. & Kiessling, R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 70, 4335–4345 (2010).
Walkley, C.R., Shea, J.M., Sims, N.A., Purton, L.E. & Orkin, S.H. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell 129, 1081–1095 (2007).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010).
Borregaard, N. & Cowland, J.B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89, 3503–3521 (1997).
Varol, C. et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J. Exp. Med. 204, 171–180 (2007).
Sasmono, R.T. et al. Mouse neutrophilic granulocytes express mRNA encoding the macrophage colony-stimulating factor receptor (CSF-1R) as well as many other macrophage-specific transcripts and can transdifferentiate into macrophages in vitro in response to CSF-1. J. Leukoc. Biol. 82, 111–123 (2007).
Burkhart, D.L. & Sage, J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat. Rev. Cancer 8, 671–682 (2008).
Giacinti, C. & Giordano, A. RB and cell cycle progression. Oncogene 25, 5220–5227 (2006).
Calo, E. et al. Rb regulates fate choice and lineage commitment in vivo. Nature 466, 1110–1114 (2010).
Macleod, K.F. The role of the RB tumour suppressor pathway in oxidative stress responses in the haematopoietic system. Nat. Rev. Cancer 8, 769–781 (2008).
Traore, K. et al. Signal transduction of phorbol 12-myristate 13-acetate (PMA)-induced growth inhibition of human monocytic leukemia THP-1 cells is reactive oxygen dependent. Leuk. Res. 29, 863–879 (2005).
Bergh, G., Ehinger, M., Olsson, I., Jacobsen, S.E. & Gullberg, U. Involvement of the retinoblastoma protein in monocytic and neutrophilic lineage commitment of human bone marrow progenitor cells. Blood 94, 1971–1978 (1999).
Daria, D. et al. The retinoblastoma tumor suppressor is a critical intrinsic regulator for hematopoietic stem and progenitor cells under stress. Blood 111, 1894–1902 (2008).
Walkley, C.R. & Orkin, S.H. Rb is dispensable for self-renewal and multilineage differentiation of adult hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 103, 9057–9062 (2006).
Viatour, P. et al. Hematopoietic stem cell quiescence is maintained by compound contributions of the retinoblastoma gene family. Cell Stem Cell 3, 416–428 (2008).
Fajas, L. et al. The retinoblastoma-histone deacetylase 3 complex inhibits PPARgamma and adipocyte differentiation. Dev. Cell 3, 903–910 (2002).
Zhang, H.S. et al. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell 101, 79–89 (2000).
Takaki, T., Fukasawa, K., Suzuki-Takahashi, I. & Hirai, H. Cdk-mediated phosphorylation of pRB regulates HDAC binding in vitro. Biochem. Biophys. Res. Commun. 316, 252–255 (2004).
Fan, L.X., Li, X., Magenheimer, B., Calvet, J.P. & Li, X. Inhibition of histone deacetylases targets the transcription regulator Id2 to attenuate cystic epithelial cell proliferation. Kidney Int. 81, 76–85 (2012).
Gutsch, R. et al. CCAAT/enhancer-binding protein beta inhibits proliferation in monocytic cells by affecting the retinoblastoma protein/E2F/cyclin E pathway but is not directly required for macrophage morphology. J. Biol. Chem. 286, 22716–22729 (2011).
Lasorella, A., Rothschild, G., Yokota, Y., Russell, R.G. & Iavarone, A. Id2 mediates tumor initiation, proliferation, and angiogenesis in Rb mutant mice. Mol. Cell. Biol. 25, 3563–3574 (2005).
Ji, H. et al. Kras activation generates an inflammatory response in lung tumors. Oncogene 25, 2105–2112 (2006).
Pickard, A., Wong, P.P. & McCance, D.J. Acetylation of Rb by PCAF is required for nuclear localization and keratinocyte differentiation. J. Cell Sci. 123, 3718–3726 (2010).
Acknowledgements
We thank A. Beg (H. Lee Moffitt Cancer Center) for providing us with Kras/mCC10 mice and D.J. McCance (Queen's University Belfast, UK) for providing us with Ad-Rb1 vectors. This work was supported by US National Institutes of Health grant CA84488.
Author information
Authors and Affiliations
Contributions
J.-I.Y. participated in design and performed most of the experiments evaluating MDSCs, V.K. and M.C. did most of the experiments assessing Rb1; Y.N., T.C., P.C. performed some of the experiments; A.V. assisted in experiments with HDAC and provided advice; P.H. performed cytological evaluation of the samples; S.A., J.C.M., M.F., A.S. and E.S. provided human samples and advice. D.I.G. designed most of the experiments, analyzed the data and together with J.-I.Y., Y.N. and T.C. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Table 1 (PDF 965 kb)
Rights and permissions
About this article
Cite this article
Youn, JI., Kumar, V., Collazo, M. et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol 14, 211–220 (2013). https://doi.org/10.1038/ni.2526
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2526
This article is cited by
-
Tumor-associated myeloid cells in cancer immunotherapy
Journal of Hematology & Oncology (2023)
-
Myeloid-derived suppressor cells: key immunosuppressive regulators and therapeutic targets in hematological malignancies
Biomarker Research (2023)
-
CD115− monocytic myeloid-derived suppressor cells are precursors of OLFM4high polymorphonuclear myeloid-derived suppressor cells
Communications Biology (2023)
-
Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets
Journal of Hematology & Oncology (2022)
-
Myeloid-derived suppressor cells in hematologic malignancies: two sides of the same coin
Experimental Hematology & Oncology (2022)