Abstract
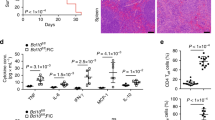
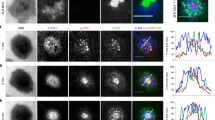
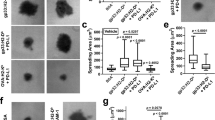
Regulatory T (Treg) cells, which maintain immune homeostasis and self-tolerance, form an immunological synapse (IS) with antigen-presenting cells (APCs). However, signaling events at the Treg cell IS remain unknown. Here we show that the kinase PKC-η associated with CTLA-4 and was recruited to the Treg cell IS. PKC-η–deficient Treg cells displayed defective suppressive activity, including suppression of tumor immunity but not of autoimmune colitis. Phosphoproteomic and biochemical analysis revealed an association between CTLA-4–PKC-η and the GIT2-αPIX-PAK complex, an IS-localized focal adhesion complex. Defective activation of this complex in PKC-η–deficient Treg cells was associated with reduced depletion of CD86 from APCs by Treg cells. These results reveal a CTLA-4–PKC-η signaling axis required for contact-dependent suppression and implicate this pathway as a potential cancer immunotherapy target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
02 May 2014
In the version of this article initially published, the second affiliation for Nicholas R.J. Gascoigne was incorrect. The correct affiliation is The Department of Microbiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore. The error has been corrected in the HTML and PDF versions of the article.
References
Bennett, C.L. et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27, 20–21 (2001).
Fontenot, J.D., Gavin, M.A. & Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Zanin-Zhorov, A. et al. Protein kinase C-theta mediates negative feedback on regulatory T cell function. Science 328, 372–376 (2010).
Collins, A.V. et al. The interaction properties of costimulatory molecules revisited. Immunity 17, 201–210 (2002).
Yokosuka, T. et al. Spatiotemporal basis of CTLA-4 costimulatory molecule-mediated negative regulation of T cell activation. Immunity 33, 326–339 (2010).
Teft, W.A., Kirchhof, M.G. & Madrenas, J. A molecular perspective of CTLA-4 function. Annu. Rev. Immunol. 24, 65–97 (2006).
Kong, K.F. & Altman, A. In and out of the bull's eye: protein kinase Cs in the immunological synapse. Trends Immunol. 34, 234–242 (2013).
Kong, K.F. et al. A motif in the V3 domain of the kinase PKC-theta determines its localization in the immunological synapse and functions in T cells via association with CD28. Nat. Immunol. 12, 1105–1112 (2011).
Kim, J.K. et al. Impact of the TCR signal on regulatory T cell homeostasis, function, and trafficking. PLoS ONE 4, e6580 (2009).
Park, S.G. et al. T regulatory cells maintain intestinal homeostasis by suppressing gammadelta T cells. Immunity 33, 791–803 (2010).
Chuck, M.I., Zhu, M., Shen, S. & Zhang, W. The role of the LAT-PLC-gamma1 interaction in T regulatory cell function. J. Immunol. 184, 2476–2486 (2010).
Spitaler, M., Emslie, E., Wood, C.D. & Cantrell, D. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity 24, 535–546 (2006).
Wing, K. et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275 (2008).
Qureshi, O.S. et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 332, 600–603 (2011).
Haribhai, D. et al. Regulatory T cells dynamically control the primary immune response to foreign antigen. J. Immunol. 178, 2961–2972 (2007).
Tang, Q. & Bluestone, J.A. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat. Immunol. 9, 239–244 (2008).
Josefowicz, S.Z., Lu, L.F. & Rudensky, A.Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Matsuda, J.L. et al. Systemic activation and antigen-driven oligoclonal expansion of T cells in a mouse model of colitis. J. Immunol. 164, 2797–2806 (2000).
Collison, L.W. et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450, 566–569 (2007).
Shimizu, J., Yamazaki, S. & Sakaguchi, S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 163, 5211–5218 (1999).
Fu, G. et al. Protein kinase C eta is required for T cell activation and homeostatic proliferation. Sci. Signal. 4, ra84 (2011).
Tai, X. et al. Basis of CTLA-4 function in regulatory and conventional CD4+ T cells. Blood 119, 5155–5163 (2012).
Kataoka, H. et al. CD25(+)CD4(+) regulatory T cells exert in vitro suppressive activity independent of CTLA-4. Int. Immunol. 17, 421–427 (2005).
Onishi, Y., Fehervari, Z., Yamaguchi, T. & Sakaguchi, S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl. Acad. Sci. USA 105, 10113–10118 (2008).
Tran, D.Q. et al. Analysis of adhesion molecules, target cells, and role of IL-2 in human FOXP3+ regulatory T cell suppressor function. J. Immunol. 182, 2929–2938 (2009).
Sarris, M., Andersen, K.G., Randow, F., Mayr, L. & Betz, A.G. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity 28, 402–413 (2008).
Borsellino, G. et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110, 1225–1232 (2007).
Zhao, Z.S., Manser, E., Loo, T.H. & Lim, L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol. Cell. Biol. 20, 6354–6363 (2000).
Lucanic, M. & Cheng, H.J.A. RAC/CDC-42-independent GIT/PIX/PAK signaling pathway mediates cell migration in C. elegans. PLoS Genet. 4, e1000269 (2008).
Phee, H., Abraham, R.T. & Weiss, A. Dynamic recruitment of PAK1 to the immunological synapse is mediated by PIX independently of SLP-76 and Vav1. Nat. Immunol. 6, 608–617 (2005).
Ku, G.M., Yablonski, D., Manser, E., Lim, L. & Weiss, A.A. A Pak1-PIX-PKL complex is activated by the T-cell receptor independent of Nck, Slp-76 and LAT. EMBO J. 20, 457–465 (2001).
Uhlig, H.H. et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J. Immunol. 177, 5852–5860 (2006).
Dustin, M.L. T-cell activation through immunological synapses and kinapses. Immunol. Rev. 221, 77–89 (2008).
Walker, L.S. & Sansom, D.M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 11, 852–863 (2011).
Read, S., Malmström, V. & Powrie, F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of Cd25+Cd4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192, 295–302 (2000).
Tivol, E.A. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 (1995).
Waterhouse, P. et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985–988 (1995).
Buhlmann, J.E., Elkin, S.K. & Sharpe, A.H. A role for the B7–1/B7–2:CD28/CTLA-4 pathway during negative selection. J. Immunol. 170, 5421–5428 (2003).
Ise, W. et al. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat. Immunol. 11, 129–135 (2010).
Schneider, H. et al. Reversal of the TCR stop signal by CTLA-4. Science 313, 1972–1975 (2006).
Rudd, C.E. The reverse stop-signal model for CTLA4 function. Nat. Rev. Immunol. 8, 153–160 (2008).
Lu, Y., Schneider, H. & Rudd, C.E. Murine regulatory T cells differ from conventional T cells in resisting the CTLA-4 reversal of TCR stop-signal. Blood 120, 4560–4570 (2012).
Peggs, K.S., Quezada, S.A. & Allison, J.P. Cancer immunotherapy: co-stimulatory agonists and co-inhibitory antagonists. Clin. Exp. Immunol. 157, 9–19 (2009).
Peggs, K.S., Quezada, S.A., Chambers, C.A., Korman, A.J. & Allison, J.P. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 206, 1717–1725 (2009).
Kim, G. et al. Spontaneous colitis occurrence in transgenic mice with altered B7-mediated costimulation. J. Immunol. 181, 5278–5288 (2008).
Becart, S. et al. SLAT regulates Th1 and Th2 inflammatory responses by controlling Ca2+/NFAT signaling. J. Clin. Invest. 117, 2164–2175 (2007).
Wang, H. et al. The interaction of CtIP and Nbs1 connects CDK and ATM to regulate HR-mediated double-strand break repair. PLoS Genet. 9, e1003277 (2013).
Washburn, M.P., Wolters, D. & Yates, J.R. III. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat. Biotechnol. 19, 242–247 (2001).
Beausoleil, S.A., Villen, J., Gerber, S.A., Rush, J. & Gygi, S.P. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 24, 1285–1292 (2006).
Lu, B., Ruse, C., Xu, T., Park, S.K. & Yates, J. III. Automatic validation of phosphopeptide identifications from tandem mass spectra. Anal. Chem. 79, 1301–1310 (2007).
Acknowledgements
We acknowledge H. Cheroutre and Y.-C. Liu for helpful discussions, the excellent services provided by members of the Flow Cytometry Core Unit at the La Jolla Institute for Allergy and Immunology, as well as the Animal Husbandry Units at LIAI and the Scripps Research Institute. This is publication number 1552 from the La Jolla Institute for Allergy and Immunology and 21923 from The Scripps Research Institute. This work was supported by US National Institutes of Health grants CA35299 (A.A.), GM065230 (N.R.J.G.) and P01AI089624 (M.K.). K.-F.K. was supported by LIAI-T1D-CRF 2012 Fellowship and Young Investigator Award #270056 from the Melanoma Research Alliance.
Author information
Authors and Affiliations
Contributions
K.-F.K. and G.F. designed experiments, collected data, performed analyses and wrote the paper; J.C., T.Y. and T.S. performed microscopy experiment; A.J.C.-B. was involved in experiments and data collection; S.B. performed and assisted in melanoma studies; Y.Z. and J.R.Y. did the phosphoproteomic experiments and analyses; G.K. and M.K. provided critical reagents and were involved in study design; N.R.J.G. and A.A. designed the study, analyzed data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Lack of CTLA-4 interaction with PKC isoforms other than PKC-η, and the expression of PKC-η and CTLA-4 in Treg vs. Teff cells.
(a) MCC-specific T hybridoma cells were left unstimulated (-) or stimulated (+) for 5 min with crosslinked anti-CD3 plus -CTLA-4 mAbs. Cell lysates (WCL) or CTLA-4 immunoprecipitates (2 left lanes in each panel) were resolved by SDS-PAGE, and immunoblotted with the indicated PKC-specific or anti-CTLA-4 Abs. (b) CD4+ T cells were purified from spleens of FIG mice, and FACS-sorted into GFP+ (Treg) and GFP− (Teff) cells. Equal number of sorted cells were subjected to RNA purification, reverse transcription, and quantitative PCR to determine the mRNA levels of Prkch and Ctla4 (left panel). Intracellular staining of PKC-η and CTLA-4 was performed to determine their respective protein levels (right panel).
Supplementary Figure 2 Frequency of Treg cell populations.
(a) The CD4+Foxp3+ cell population from thymi, spleens, peripheral lymph nodes (pLN) and mesenteric lymph nodes (mLN) of 8- to 12-week-old mice were determined by intracellular staining of Foxp3. (b) Number of GFP+ Treg cells recovered from mice undergoing homeostatic expansion (see Fig. 3b-d). Naïve T cells from allotypically marked CD45.1+ B6.SJL mice were transferred alone (None), or cotransferred with FACS-sorted CD4+GFP+ Treg cells from WT or Prkch−/− FIG mice into Rag1−/− mice. The numbers of GFP+ cells in spleens, pLN and mLN were determined. Each data point represents a single mouse.
Supplementary Figure 3 Prkch−/− Treg cells protect mice in a T cell transfer model of colitis.
(a) Sorted CD4+CD62L+ naïve T cells (Teff) in the absence or presence of WT or Prkch−/− GFP+ Treg cells were cotransferred into Rag1−/− mice and weight was monitored over time as indicated. Mice were sacrificed 10 weeks post-transfer. (b,c) The infiltrating T cell populations in spleens, peripheral lymph nodes (pLN) and mesenteric lymph nodes (mLN) were analyzed by flow cytometry and enumerated. **P < 0.05.
Supplementary Figure 4 Conservation of the membrane-proximal basic CTLA-4 motif and its importance in PKC-h association.
(a) Evolutionary conservation of the positively charged proximal motif (underlined, with basic residues in bold) in the cytoplamic tail of CTLA-4. Protein sequence of putative CTLA-4 proteins from the indicated organisms were aligned with human CTLA-4. The consensus sequence was generated using Weblogo (www.weblogo.berkeley.edu). (b) . Analysis of “tailess” CTLA-4 mutants for their interaction with PKC-η. WT or truncated CTLA-4 were cotransfected with Xpress-tagged WT PKC-η into JTAg cells. Cells were stimulated with anti-CD3 mAbs + CD86-Fc recombinant protein for 5 min and immunoprecipitated with an anti-CTLA-4 mAb prior to immunoblotting. The right panel shows a schematic representation of mouse CTLA-4 and its cytoplasmic tail. The CTLA-4 tail was partially (Δ192-223) or fully (Δ182-223) truncated.
Supplementary Figure 5 Effect of Prkch deletion on LFA-1 function.
Purified CD4+ cells from WT or Prkch-/- FIG mice were stimulated with anti-CD3 plus anti-CTLA-4 Abs for the indicated times. The function of LFA-1 was measured by its ability to bind to ICAM1-Fc. Cells were stained with fluorophore-conjugated anti-CD4 and anti-Fc antibodies. Shown are representative data gated on GFP+ cells (top panel) and cumulative data of 2 independent experiments (bottom panel).
Supplementary Figure 6 Phosphoproteome analysis of CD3- plus CTLA-4-costimulated Treg cells and signal requirements for recruitment of the GIT-PIX-PAK complex to CTLA-4.
(a) Purified CD4+ T cells from WT or Prkch−/− FIG mice were differentiated for 6 days into iTregs in the presence of TGF-β and IL-2 in standard SILAC media. The cells were stimulated with anti-CD3 plus anti-CTLA-4 mAbs for 5 min before cell lysis and sample preparation for phosphoproteomic analysis. Shown are representative hypophosphorylated proteins in Prkch−/− Tregs as compared to WT Tregs with a fold-change of >1.5. (b) Recruitment of GIT-PIX-PAK complex to CTLA-4-PKC-h complex is dependent on CTLA-4, but not CD28. MCC-specific T hybridoma cells were left unstimulated or stimulated with anti-CD3 and anti-B7, anti-CD3 and anti-CD28, or anti-CD3 and anti-CTLA-4 for 5 min prior to CTLA-4 immunoprecipitation. Immunoblotting was carried out with indicated antibodies.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 1355 kb)
Rights and permissions
About this article
Cite this article
Kong, KF., Fu, G., Zhang, Y. et al. Protein kinase C-η controls CTLA-4–mediated regulatory T cell function. Nat Immunol 15, 465–472 (2014). https://doi.org/10.1038/ni.2866
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2866
This article is cited by
-
Anti-CTLA-4 nanobody as a promising approach in cancer immunotherapy
Cell Death & Disease (2024)
-
Insights from a 30-year journey: function, regulation and therapeutic modulation of PD1
Nature Reviews Immunology (2023)
-
Immune checkpoint inhibitors and cancer immunotherapy by aptamers: an overview
Medical Oncology (2023)
-
Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer
Journal of Experimental & Clinical Cancer Research (2021)
-
Intratumoural administration and tumour tissue targeting of cancer immunotherapies
Nature Reviews Clinical Oncology (2021)