Abstract
Deubiquitinases (DUBs) are a new class of drug targets, although the physiological function of only few DUBs has been characterized. Here we identified the DUB USP15 as a crucial negative regulator of T cell activation. USP15 stabilized the E3 ubiquitin ligase MDM2, which in turn negatively regulated T cell activation by targeting the degradation of the transcription factor NFATc2. USP15 deficiency promoted T cell activation in vitro and enhanced T cell responses to bacterial infection and tumor challenge in vivo. USP15 also stabilized MDM2 in cancer cells and regulated p53 function and cancer-cell survival. Our results suggest that inhibition of USP15 may both induce tumor cell apoptosis and boost antitumor T cell responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Liu, Y.C., Penninger, J. & Karin, M. Immunity by ubiquitylation: a reversible process of modification. Nat. Rev. Immunol. 5, 941–952 (2005).
Lipkowitz, S. & Weissman, A.M. RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat. Rev. Cancer 11, 629–643 (2011).
Hershko, A. & Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 (1998).
Chen, Z.J. & Sun, L.J. Nonproteolytic functions of ubiquitin in cell signaling. Mol. Cell 33, 275–286 (2009).
Kulathu, Y. & Komander, D. Atypical ubiquitylation - the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat. Rev. Mol. Cell Biol. 13, 508–523 (2012).
Li, W. et al. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle's dynamics and signaling. PLoS ONE 3, e1487 (2008).
Li, Q. & Lozano, G. Molecular pathways: targeting Mdm2 and Mdm4 in cancer therapy. Clin. Cancer Res. 19, 34–41 (2013).
Wade, M., Li, Y.C. & Wahl, G.M. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat. Rev. Cancer 13, 83–96 (2013).
Shangary, S. & Wang, S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu. Rev. Pharmacol. Toxicol. 49, 223–241 (2009).
Reyes-Turcu, F.E., Ventii, K.H. & Wilkinson, K.D. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu. Rev. Biochem. 78, 363–397 (2009).
Komander, D., Clague, M.J. & Urbe, S. Breaking the chains: structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 10, 550–563 (2009).
Sun, S.C. Deubiquitylation and regulation of the immune response. Nat. Rev. Immunol. 8, 501–511 (2008).
Fraile, J.M., Quesada, V., Rodriguez, D., Freije, J.M. & Lopez-Otin, C. Deubiquitinases in cancer: new functions and therapeutic options. Oncogene 31, 2373–2388 (2012).
Harhaj, E.W. & Dixit, V.M. Regulation of NF-kappaB by deubiquitinases. Immunol. Rev. 246, 107–124 (2012).
Baker, R.T., Wang, X.W., Woollatt, E., White, J.A. & Sutherland, G.R. Identification, functional characterization, and chromosomal localization of USP15, a novel human ubiquitin-specific protease related to the UNP oncoprotein, and a systematic nomenclature for human ubiquitin-specific proteases. Genomics 59, 264–274 (1999).
Vos, R.M., Altreuter, J., White, E.A. & Howley, P.M. The ubiquitin-specific peptidase USP15 regulates human papillomavirus type 16 E6 protein stability. J. Virol. 83, 8885–8892 (2009).
Inui, M. et al. USP15 is a deubiquitylating enzyme for receptor-activated SMADs. Nat. Cell Biol. 13, 1368–1375 (2011).
Eichhorn, P.J. et al. USP15 stabilizes TGF-beta receptor I and promotes oncogenesis through the activation of TGF-beta signaling in glioblastoma. Nat. Med. 18, 429–435 (2012).
Hetfeld, B.K. et al. The zinc finger of the CSN-associated deubiquitinating enzyme USP15 is essential to rescue the E3 ligase Rbx1. Curr. Biol. 15, 1217–1221 (2005).
Wakil, A.E., Wang, Z.E., Ryan, J.C., Fowell, D.J. & Locksley, R.M. Interferon gamma derived from CD4(+) T cells is sufficient to mediate T helper cell type 1 development. J. Exp. Med. 188, 1651–1656 (1998).
Smith-Garvin, J.E., Koretzky, G.A. & Jordan, M.S. T cell activation. Annu. Rev. Immunol. 27, 591–619 (2009).
Kiani, A. et al. Regulation of interferon-gamma gene expression by nuclear factor of activated T cells. Blood 98, 1480–1488 (2001).
Macian, F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 5, 472–484 (2005).
Yoeli-Lerner, M. et al. Akt blocks breast cancer cell motility and invasion through the transcription factor. NFAT. Mol. Cell 20, 539–550 (2005).
Harper, S., Besong, T.M., Emsley, J., Scott, D.J. & Dreveny, I. Structure of the USP15 N-terminal domains: a beta-hairpin mediates close association between the DUSP and UBL domains. Biochemistry 50, 7995–8004 (2011).
Kitagaki, J., Agama, K.K., Pommier, Y., Yang, Y. & Weissman, A.M. Targeting tumor cells expressing p53 with a water-soluble inhibitor of Hdm2. Mol. Cancer Ther. 7, 2445–2454 (2008).
Manfredi, J.J. The Mdm2-p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressor. Genes Dev. 24, 1580–1589 (2010).
Vassilev, L.T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004).
Ovaa, H. et al. Activity-based ubiquitin-specific protease (USP) profiling of virus-infected and malignant human cells. Proc. Natl. Acad. Sci. USA 101, 2253–2258 (2004).
Take, Y., Kumano, M., Teraoka, H., Nishimura, S. & Okuyama, A. DNA-dependent protein kinase inhibitor (OK-1035) suppresses p21 expression in HCT116 cells containing wild-type p53 induced by adriamycin. Biochem. Biophys. Res. Commun. 221, 207–212 (1996).
Cheok, C.F., Kua, N., Kaldis, P. & Lane, D.P. Combination of nutlin-3 and VX-680 selectively targets p53 mutant cells with reversible effects on cells expressing wild-type p53. Cell Death Differ. 17, 1486–1500 (2010).
Overwijk, W.W. & Restifo, N.P. B16 as a mouse model for human melanoma. Curr. Protoc. Immunol. 20, 21 (2001).
Kennedy, R. & Celis, E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol. Rev. 222, 129–144 (2008).
Li, M. et al. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416, 648–653 (2002).
Kon, N. et al. Inactivation of HAUSP in vivo modulates p53 function. Oncogene 29, 1270–1279 (2010).
Loch, C.M. & Strickler, J.E. A microarray of ubiquitylated proteins for profiling deubiquitylase activity reveals the critical roles of both chain and substrate. Biochim. Biophys. Acta 1823, 2069–2078 (2012).
Vanneman, M. & Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 12, 237–251 (2012).
Montes de Oca Luna, R., Wagner, D.S. & Lozano, G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 378, 203–206 (1995).
Chang, M. et al. The ubiquitin ligase Peli1 negatively regulates T cell activation and prevents autoimmunity. Nat. Immunol. 12, 1002–1009 (2011).
Reiley, W.W. et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J. Exp. Med. 204, 1475–1485 (2007).
Sun, S.-C., Ganchi, P.A., Beraud, C., Ballard, D.W. & Greene, W.C. Autoregulation of the NF-κB transactivator Rel A (p65) by multiple cytoplasmic inhibitors containing ankyrin motifs. Proc. Natl. Acad. Sci. USA 91, 1346–1350 (1994).
Hassink, G.C. et al. The ER-resident ubiquitin-specific protease 19 participates in the UPR and rescues ERAD substrates. EMBO Rep. 10, 755–761 (2009).
Lu, Y. et al. Th9 cells promote antitumor immune responses in vivo. J. Clin. Invest. 122, 4160–4171 (2012).
Cvijic, M.E., Xiao, G. & Sun, S.C. Study of T-cell signaling by somatic cell mutagenesis and complementation cloning. J. Immunol. Methods 278, 293–304 (2003).
Foulds, K.E. et al. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 168, 1528–1532 (2002).
Edwards, L.A. et al. Inhibition of ILK in PTEN-mutant human glioblastomas inhibits PKB/Akt activation, induces apoptosis, and delays tumor growth. Oncogene 24, 3596–3605 (2005).
Acknowledgements
We thank W. Gu for expression vectors, B. Vogelstein for HCT116 derivative cell lines, S. Hao for recombinant L. monocytogenesis, Q. Yi for B16 cell line, L.A. Donehower for Trp53−/− mice, and the personnel from the US National Institutes of Health or National Cancer Insitute–supported resources (flow cytometry, DNA analysis and animal facilities) under award number P30CA016672 at The MD Anderson Cancer Center. This study was supported by grants from US National Institutes of Health (AI057555, AI064639, GM84459 and AI104519 to S.-C.S. and AI098099 to S.S.W.) and partially supported by a Sister Institution Network Fund and a seed fund from the Center for Inflammation and Cancer at the MD Anderson Cancer Center.
Author information
Authors and Affiliations
Contributions
Q.Z. designed and performed the experiments, prepared the figures and wrote part of the manuscript; J.J., H.H., H.S.L., S.R., Y.X., M.N., X.Z., X.C., P.Y. and C.Z. contributed to the performance of the experiments; S.S.W. and G.L. were involved in supervision of H.S.L. and P.Y., respectively; S.E.U. was involved in collaboration on cancer model studies, and S.-C.S. supervised the work and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Generation and initial characterization of Usp15−/− mice.
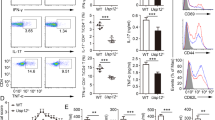
(a) Schematic picture of Usp15 gene targeting using the OmniBank retroviral gene-trapping technique. (b-d) Genotyping PCR analysis of tail DNAs (b), RT-PCR analysis of the indicated regions of Usp15 cDNA in T cells (c), and USP15 immunoblot using T cells (d) of WT (+/+), heterozygous (+/−), and Usp15−/− (−/−) mice. (e) Survival curve of WT and Usp15−/− mice. (f) Flow cytometric analysis of thymocytes from WT and Usp15−/− mice (6 wk old), showing the percentage of CD4−CD8− double negative, CD4+CD8+ double positive, and the CD4+ and CD8+ single positive populations (n=5; showing a representative plot). (g) Flow cytometric analysis of the frequency of CD3+ T cells and the CD19+ B cells in the splenocytes of WT and Usp15−/− mice (8-12 wk old) (n=5; showing a representative plot). (h,i) Flow cytometric analysis of the frequency of naïve (CD44loCD62Lhi) and memory (CD44hiCD62Llo) populations in the splenic CD4+ T cells from WT or Usp15−/− mice of 3 (h) or 10 (i) months of age (n=5; showing a representative plot). Data are representative of three (f-i) independent experiments.
Supplementary Figure 2 Effect of USP15 deficiency on CD4+ T cell activation and differentiation.
(a,b) Rag1−/− mice were adoptively transferred with WT or Usp15−/− bone marrow cells transduced with a GFP-expressing lentiviral vector (EV) or the same vector encoding USP15 or a catalytically inactive USP15 mutant (C298A). GFP+ thymocytes (a) and splenic naïve CD4+ T cells (b) were sorted from the indicated chimeric mice and subjected to USP15 IB (a) and intracellular IFN-γ staining following stimulation with anti-CD3 plus anti-CD28 for 72 h (b) (n=3; showing a representative plot). (c-e) Naïve CD4+ T cells from WT or Usp15−/− (KO) mice were stimulated under standard TH0, TH1, TH2, TH17 or iTreg conditions and harvested on day 5 for qRT-PCR (c), proliferation (d), and apoptosis assays (e) (n=3). (f) Flow cytometric analysis of Treg cells differentiated for 5 days from WT or Usp15-deficient naive CD4+ T cells under Treg cell conditions with different doses of TGF-β in the presence (+) or absence (−) of an anti-IFN-γ blocking antibody. (g) Flow cytometric analysis of the frequency of Foxp3+ Treg cells among CD3+CD4+CD8- cells in the indicated lymphoid organs of WT or Usp15−/− mice (5 wk old). Data are presented as a representative plot (left) and a summary graph (right) (n=6). All summary graphs are mean ± s.e.m and data are representative of three independent experiments. * P <0.05.
Supplementary Figure 3 USP15 deficiency enhanced T cell response to listeria infection.
(a,b) Flow cytometric analyses of the number of IFN-γ-producing CD4+ T cells (a) and the IFN-γ MFI in the IFN-γ+CD4+ T cells (b) from the spleen of wild-type and Usp15−/− mice infected with L. monocytogenes for 6 days (n=5). (c-e) Tcrb−/−Tcrd−/− mice were reconstituted with 5 × 106 wild-type or Usp15−/− (KO) naïve CD4+ T cells in the presence of equal numbers of wild-type CD8+ T cells. After 4 days, the recipient mice were sacrificed for visualizing spleen size (c, n=3), flow cytometric analysis of splenic CD4+ T cells (d, n=5), and ELISA of serum IFN-γ concentration (e, n=5). (f, g) Spleen size (f, n=3) and flow cytometric analysis of the frequency of CD11b+ monocytes and CD11c+ dendritic cells in splenocytes (g, n=5) of the reconstituted Tcrb−/−Tcrd−/− mice, infected i.v. with 5 × 104 CFU of L. monocytogenes for 4 days. wild-type or Usp15−/−, Tcrb−/−Tcrd−/− mice reconstituted with wild-type or Usp15−/− naïve CD4+ T cells (c-g). (h,i) B6.SJL mice, adoptively transferred with wild-type OT-II or Usp15−/− OT-II naïve CD4+ T cells, were either uninfected (UI) or infected for 6 days with OVA-expressing L. monocytogenes (LM) and subjected to flow cytometric analyses of the number of the total transferred T cells (CD45.2+) (h) and Treg cells (Foxp3+CD45.2+) (i) in the spleen (n=3 for uninfected and 5 for LM-infected mice). Wild-type OT-II or KO OT-II, B6.SJL mice adoptively transferred with wild-type OT-II or Usp15−/− OT-II naïve CD4+ T cells (h, i). UI, uninfected. LM, L. monocytogenes infected. Data are presented as mean ± s.e.m. and representative of at least three independent experiments. * P<0.05; ** P<0.01.
Supplementary Figure 4 USP15 was dispensable for TCR-proximal signaling and TGF-β signaling in T cells.
(a,b) IB analysis of the indicated phosphorylated (p-) and total proteins in the whole-cell lysates of naive CD4+ T cells stimulated with anti-CD3 and anti-CD28 antibodies using a crosslinking protocol. (c,d) wild-type or Usp15−/− naïve CD4+ T cells were stimulated with anti-CD3 plus anti-CD28 for 24 h, starved for 24 h, and then stimulated with TGF-β for the indicated time periods. Cells were subjected to IB (c) or qRT-PCR analysis (d) (n=3). (e,f) qRT-PCR analysis of Il2 and Ifng mRNA (e) and ELISA of secreted IL-2 and IFN-γ (f) in naïve CD4+ T cells stimulated for 24 h (e) or 48 h (f) (n=3). (g) IB analysis using cytoplasmic (CE) and nuclear (NE) extracts of naive CD4+ T cells stimulated with anti-CD3 plus anti-CD28 in the presence (+) or absence (−) of TGF-β. (h) IB analysis of NFATc2 in EL4 T cells infected with a retroviral vector (EV) or the same vector encoding NFATc2. (i,j) Intracellular staining of IFN-γ (i, n=5; showing a representative plot) and ELISA of IL-2 and IFN-γ (j, n=3) in the EL4 cells described in h, stimulated as indicated. (k) qRT-PCR analysis of Nfatc2 mRNA relative level (normalized to the control Actb) in wild-type or Usp15−/− naïve CD4+ T cells, stimulated as indicated (n=3). Data in graphs are presented as mean ± s.e.m, and representative of at least three independent experiments. * P<0.05.
Supplementary Figure 5 MDM2 associated with USP15 and regulated TCR/CD28-stimulated NFATc2 activation and cytokine induction in a p53-independent manner.
(a) MDM2-USP15 co-IP (upper) and direct IB (lower) assays using cytoplasmic (CE) and nuclear (NE) extracts of wild-type naïve CD4+ T cells, incubated for 2 h with MG132 either in the absence (−) or presence of anti-CD3 plus anti-CD28. (b) IB analysis using cytoplasmic (CE) and nuclear (NE) extracts of untreated (UT) wild-type or Usp15−/− naïve CD4+ T cells. (c) IB analysis using cytoplasmic (CE) and nuclear (NE) extracts of wild-type naive CD4+ T cells incubated with (+) or without (−) anti-CD3 plus anti-CD28 for 3 h in the presence of MG132 during the last 2 h. (d) NFATc2 ubiquitination (upper) and IB (lower) analyses using HEK293 cells transfected with the indicated expression vectors. (e,f) IB (e) and qRT-PCR (f, n=3) analyses in naïve CD4+ T cells stimulated with anti-CD3 plus anti-CD28 in the absence (−) or presence of HLI373 or DMSO. (g-i) IB analyses using GFP+ thymocytes (g) or naïve CD4+ T cells (h) and IFN-γ ICS in stimulated GFP+ naïve CD4+ T cells (n=5; showing a representative plot) of Rag1−/− mice adoptively transferred (for 6 wk) with Usp15−/− bone marrow cells transduced with a GFP-expressing vector (EV) or the same vector encoding HA-tagged MDM2. (j) IB analysis using sorted (GFP+) wild-type or Usp15−/− naïve CD4+ T cells reconstituted, via bone marrow adoptive transfer, with a GFP-expressing vector (EV), USP15, or USP15C298A and stimulated as indicated. (k,l) qRT-PCR (k) and IB (l) analyses of wild-type naive CD4+ T cells stimulated with anti-CD3 plus anti-CD28 in the presence of DMSO or Nutlin-3 (n=3). Data in summary graphs are mean ± s.e.m, and representative of at least three independent experiments. * P<0.05.
Supplementary Figure 6 CD8+ T cells expressed lower levels of MDM2 and were not influenced by USP15 deficiency in cytokine production.
(a) IB analysis of USP15 and MDM2 protein levels in CD4+ and CD8+ T cells. A nonspecific band is indicated as NS. (b) qRT-PCR analysis of Mdm2 and Usp15 mRNA levels in CD4+ and CD8+ T cells (n=3). (c) IB analysis of NFATc2 and loading controls in the nuclear (NE) and cytoplasmic (CE) extracts of wild-type or Usp15−/− CD8+ T cells stimulated as indicated. (d,e) ELISA (d, n=3) and qRT-PCR (e, n=3) analyses using wild-type and Usp15−/− (KO) CD8+ T cells stimulated for the indicated time periods with anti-CD3 plus anti-CD28. Data are representative of three independent experiments. NS, P>0.05, **P<0.01.
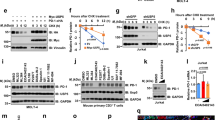
Supplementary Figure 7 USP15 was abundantly expressed in cancer cells and required for MDM2 expression and cancer cell survival.
(a) IB analysis using whole-cell extracts of melanoma and colon cancer cell lines. (b) IB analysis of the indicated proteins in human primary dermal fibroblasts transduced with pGIPZ lentiviral vectors encoding either a control shRNA (C) or two different USP15 shRNAs. (c) IB analysis of the indicated proteins using WT or Usp15−/− murine B cells and thymocytes. (d,e) Control shRNA (Ctrl)-transduced or USP15 shRNA (shUSP15)-transduced A375 cells were reconstituted with either an empty vector (EV) or an RNAi-resistant USP15 expression vector. The cells were subject to IB analysis of the indicated proteins (d) or flow cytometric analysis of spontaneous apoptosis showing a representative plot (numbers in the figure panels indicate percentage of apoptotic cells) and a summary graph (e) (n=3). (f) IB analysis of the indicated proteins using HCT116 p53 WT or HCT116 p53 null (Trp53 −/−) cells infected with control (c) or USP15 shRNAs. (g) Apoptotis analyses of the HCT116 p53 WT and HCT116 p53 null (Trp53 −/−) cells (n=3). Bar graphs are mean ± s.e.m. Data are representative of three independent experiments. *P<0.05; **P<0.01.
Supplementary Figure 8 USP15 regulated the stability of MDM2 but was dispensable for TGF-β signaling in A375 and HCT116 cells.
(a) IB analysis of MDM2 in control (C) or USP15 knockdown (shRNA 1 and shRNA 2) A375 cells incubated for 4 h with10 μg/ml MG132 or the solvent control DMSO. (b) IB analysis of the indicated proteins in control (Ctrl) or USP15 knockdown (shRNA 1) cells incubated with cycloheximide (50 μg/ml) for the indicated time periods. (c) A375 and HCT116 cells were stably infected with a non-silencing control shRNA (C) or two different USP15 shRNAs. The cells were serum-starved for 24 h and stimulated with TGF-β for 1h, and whole-cell lysates were subjected to IB analysis of the indicated phosphorylated (p-) and total proteins. (d,e) qRT-PCR analysis of Pai1 and Smad7 mRNA in the indicated cells, either untreated (UT) or stimulated with TGF-β for 4h (n=3; mean ± s.e.m.). Data are representative of three independent experiments.
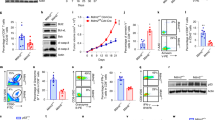
Supplementary Figure 9 USP15 regulated tumor growth and antitumor T cell responses.
(a) Growth curve of xenograft tumors in nude mice that were injected s.c. with HCT116 cells (1× 107 cells per mouse) stably expressing a control (Ctrl) shRNA or a USP15-specific shRNA (shRNA 1) (n=7). (b) IB analysis of the indicated proteins using lysates the xenograft tumors collected on day 21 post injection. (c) Growth curve of xenograft tumors in nude mice injected s.c. with USP15-silenced HCT116 p53 null (Trp53 −/−) cells (5 × 106 cells per mouse) (n=5). (d) IB analysis using control (Ctrl) or USP15-knockdown B16 tumor cells. (e,f) Growth curve of tumors in wild-type or Usp15−/− (KO) mice injected s.c. with a low dose (5 × 105 cells per mouse) (e) or a high dose (5 × 106 cells per mouse) (f) of the indicated B16 cells (n=5). (g,h) wild-type and Usp15−/− mice were injected s.c. with B16 melanoma cells. After 15 days of challenge, tumors were removed for ICS and flow cytometry analyses of the number of IFN-γ-producing tumor-infiltrating CD4+ (g) and the frequency of IFN-γ-producing tumor-infiltrating CD8+ (h) T cells. Data are presented as a representative plot or a summary graph of multiple mice (n=5). Summary graphs are mean ± s.e.m., and data are representative of three independent experiments. * P<0.05.
Supplementary Figure 10 USP15 deficiency promoted IFN-γ−dependent tumor suppression but did not influence Treg cell frequency.
(a,b) Flow cytometry analysis of the frequency of Foxp3+CD4+ T cells in the spleen, draining lymph node (dLN), and tumor-infiltrating lymphocytes (TIL) of day 15 B16-challenged wild-type and Usp15−/− mice. Data are presented as a representative plot (a) and a summary graph of multiple mice (n=10) (b). (c) Tumor growth curves in wild-type or Usp15−/− mice injected s.c. with B16 melanoma cells and administered i.p. with 0.5 mg of an anti-mouse IFN-γ neutralizing antibody or a rat IgG control every 3 days starting from day 1 of B16 challenge. (d) Flow cytometric analysis of splenic CD4+ T cells in Tcrb−/−Tcrd−/− mice reconstituted (for 16 days) with 5 × 106 wild-type or Usp15−/− (KO) naïve CD4+ T cells in the presence of equal numbers of wild-type CD8+ T cells (n=5). Summary graphs are mean ± s.e.m., and data are representative of three independent experiments. NS, P>0.05; * P<0.05.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Tables 1–5 (PDF 3163 kb)
Rights and permissions
About this article
Cite this article
Zou, Q., Jin, J., Hu, H. et al. USP15 stabilizes MDM2 to mediate cancer-cell survival and inhibit antitumor T cell responses. Nat Immunol 15, 562–570 (2014). https://doi.org/10.1038/ni.2885
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2885
This article is cited by
-
Targeting MDM2 in malignancies is a promising strategy for overcoming resistance to anticancer immunotherapy
Journal of Biomedical Science (2024)
-
TRIM21/USP15 balances ACSL4 stability and the imatinib resistance of gastrointestinal stromal tumors
British Journal of Cancer (2024)
-
USP15 promotes pulmonary vascular remodeling in pulmonary hypertension in a YAP1/TAZ-dependent manner
Experimental & Molecular Medicine (2023)
-
Deubiquitinase YOD1 suppresses tumor progression by stabilizing E3 ligase TRIM33 in head and neck squamous cell carcinoma
Cell Death & Disease (2023)
-
Ablation of the deubiquitinase USP15 ameliorates nonalcoholic fatty liver disease and nonalcoholic steatohepatitis
Experimental & Molecular Medicine (2023)