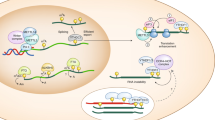
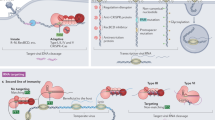
Abstract
The rapid changes in gene expression that accompany developmental transitions, stress responses and proliferation are controlled by signal-mediated coordination of transcriptional and post-transcriptional mechanisms. In recent years, understanding of the mechanics of these processes and the contexts in which they are employed during hematopoiesis and immune challenge has increased. An important aspect of this progress is recognition of the importance of RNA-binding proteins and noncoding RNAs. These have roles in the development and function of the immune system and in pathogen life cycles, and they represent an important aspect of intracellular immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Djebali, S. et al. Landscape of transcription in human cells. Nature 489, 101–108 (2012).
Cech, T.R. & Steitz, J.A. The noncoding RNA revolution—trashing old rules to forge new ones. Cell 157, 77–94 (2014).
Rinn, J.L. & Chang, H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 81, 145–166 (2012).
Chen, C.Y., Chen, S.T., Juan, H.F. & Huang, H.C. Lengthening of 3′ UTR increases with morphological complexity in animal evolution. Bioinformatics 28, 3178–3181 (2012).
Carpenter, S. et al. A long noncoding RNA mediates both activation and repression of immune response genes. Science 341, 789–792 (2013).In this article, a lncRNA regulated by MyD88 and NF-kB is found to activate or suppress distinct classes of transcripts. The lncRNA interacts with an RBP known to regulate transcription.
Rapicavoli, N.A. et al. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. eLife 2, e00762 (2013).
Hu, G. et al. Expression and regulation of intergenic long noncoding RNAs during T cell development and differentiation. Nat. Immunol. 14, 1190–1198 (2013).
Geisler, S. & Coller, J. RNA in unexpected places: long noncoding RNA functions in diverse cellular contexts. Nat. Rev. Mol. Cell Biol. 14, 699–712 (2013).
Williams, G.T., Mourtada-Maarabouni, M. & Farzaneh, F. A critical role for noncoding RNA GAS5 in growth arrest and rapamycin inhibition in human T-lymphocytes. Biochem. Soc. Trans. 39, 482–486 (2011).
Kino, T., Hurt, D.E., Ichijo, T., Nader, N. & Chrousos, G.P. Noncoding RNA Gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 3, ra8 (2010).
Vigneau, S., Rohrlich, P.S., Brahic, M. & Bureau, J.F. Tmevpg1, a candidate gene for the control of Theiler's virus persistence, could be implicated in the regulation of gamma interferon. J. Virol. 77, 5632–5638 (2003).
Gomez, J.A. et al. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cell 152, 743–754 (2013).In this article, a noncoding RNA conferring resistance to salmonella is found to regulate histone methylation at the locus encoding IFN-γ. The lncRNA interacts with components of the methyltransferase.
Bentley, D.L. Coupling mRNA processing with transcription in time and space. Nat. Rev. Genet. 15, 163–175 (2014).
Bhatt, D.M. et al. Transcript dynamics of proinflammatory genes revealed by sequence analysis of subcellular RNA fractions. Cell 150, 279–290 (2012).
Saletore, Y., Chen-Kiang, S. & Mason, C.E. Novel RNA regulatory mechanisms revealed in the epitranscriptome. RNA Biol. 10, 342–346 (2013).
Schones, D.E. et al. Dynamic regulation of nucleosome positioning in the human genome. Cell 132, 887–898 (2008).
Nagaike, T. & Manley, J.L. Transcriptional activators enhance polyadenylation of mRNA precursors. RNA Biol. 8, 964–967 (2011).
Takagaki, Y. & Manley, J.L. Levels of polyadenylation factor CstF-64 control IgM heavy chain mRNA accumulation and other events associated with B cell differentiation. Mol. Cell 2, 761–771 (1998).
Martinez, N.M. & Lynch, K.W. Control of alternative splicing in immune responses: many regulators, many predictions, much still to learn. Immunol. Rev. 253, 216–236 (2013).
Weischenfeldt, J. et al. NMD is essential for hematopoietic stem and progenitor cells and for eliminating by-products of programmed DNA rearrangements. Genes Dev. 22, 1381–1396 (2008).
Spellman, R., Llorian, M. & Smith, C.W. Crossregulation and functional redundancy between the splicing regulator PTB and its paralogs nPTB and ROD1. Mol. Cell 27, 420–434 (2007).
Sandberg, R., Neilson, J.R., Sarma, A., Sharp, P.A. & Burge, C.B. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science 320, 1643–1647 (2008).
Lianoglou, S., Garg, V., Yang, J.L., Leslie, C.S. & Mayr, C. Ubiquitously transcribed genes use alternative polyadenylation to achieve tissue-specific expression. Genes Dev. 27, 2380–2396 (2013).This article presents an improved method for determining the site of polyadenylation applied to various cell types, including B lymphocytes.
Ameres, S.L. & Zamore, P.D. Diversifying microRNA sequence and function. Nat. Rev. Mol. Cell Biol. 14, 475–488 (2013).
Wang, X. et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120 (2013).
Sedger, L.M. microRNA control of interferons and interferon induced anti-viral activity. Mol. Immunol. 56, 781–793 (2013).
Scott, D.D. & Norbury, C.J. RNA decay via 3′ uridylation. Biochim. Biophys. Acta 1829, 654–665 (2013).
Wan, Y. et al. Landscape and variation of RNA secondary structure across the human transcriptome. Nature 505, 706–709 (2014).
Rouskin, S., Zubradt, M., Washietl, S., Kellis, M. & Weissman, J.S. Genome-wide probing of RNA structure reveals active unfolding of mRNA structures in vivo. Nature 505, 701–705 (2014).
Turner, M. & Hodson, D. Regulation of lymphocyte development and function by RNA-binding proteins. Curr. Opin. Immunol. 24, 160–165 (2012).
Rabani, M. et al. Metabolic labeling of RNA uncovers principles of RNA production and degradation dynamics in mammalian cells. Nat. Biotechnol. 29, 436–442 (2011).
Friedel, C.C., Dolken, L., Ruzsics, Z., Koszinowski, U.H. & Zimmer, R. Conserved principles of mammalian transcriptional regulation revealed by RNA half-life. Nucleic Acids Res. 37, e115 (2009).
Turner, M. & Katsikis, P.D. A new mechanism of gene regulation mediated by noncoding RNA. J. Immunol. 189, 3–4 (2012).
Kontoyiannis, D., Pasparakis, M., Pizarro, T.T., Cominelli, F. & Kollias, G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10, 387–398 (1999).
Kedersha, N., Ivanov, P. & Anderson, P. Stress granules and cell signaling: more than just a passing phase? Trends Biochem. Sci. 38, 494–506 (2013).
Ramachandran, S. & Palanisamy, V. Horizontal transfer of RNAs: exosomes as mediators of intercellular communication. Wiley Interdiscip. Rev. RNA 3, 286–293 (2012).
Aucher, A., Rudnicka, D. & Davis, D.M. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J. Immunol. 191, 6250–6260 (2013).
Villarroya-Beltri, C. et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Comun. 4, 2980 (2013).This article provides an analysis of miRNA repertoire of T cell–secreted exosomes and offers important insights into the regulation of the nonrandom sorting of miRNA by RBPs.
Nolte-'t Hoen, E.N. et al. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small noncoding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 40, 9272–9285 (2012).
Huang, X. et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics 14, 319 (2013).
Ray, D. et al. A compendium of RNA-binding motifs for decoding gene regulation. Nature 499, 172–177 (2013).
Leppek, K. et al. Roquin promotes constitutive mRNA decay via a conserved class of stem-loop recognition motifs. Cell 153, 869–881 (2013).In this article, a screen for proteins interacting with a sequence that promotes RNA decay from the TNF mRNA identifies roquin, an RBP previously linked to tolerance and follicular helper T cell function.
Vinuesa, C.G. et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435, 452–458 (2005).
Vogel, K.U. et al. Roquin paralogs 1 and 2 redundantly repress the Icos and Ox40 costimulator mRNAs and control follicular helper T cell differentiation. Immunity 38, 655–668 (2013).
Pratama, A. et al. Roquin-2 shares functions with its paralog Roquin-1 in the repression of mRNAs controlling T follicular helper cells and systemic inflammation. Immunity 38, 669–680 (2013).
Minagawa, K. et al. Posttranscriptional modulation of cytokine production in T cells for the regulation of excessive inflammation by TFL. J. Immunol. 192, 1512–1524 (2014).
Hodson, D.J. et al. Deletion of the RNA-binding proteins ZFP36L1 and ZFP36L2 leads to perturbed thymic development and T lymphoblastic leukemia. Nat. Immunol. 11, 717–724 (2010).
Kedde, M. & Agami, R. Interplay between microRNAs and RNA-binding proteins determines developmental processes. Cell Cycle 7, 899–903 (2008).
Meisner, N.C. et al. mRNA openers and closers: modulating AU-rich element-controlled mRNA stability by a molecular switch in mRNA secondary structure. ChemBioChem 5, 1432–1447 (2004).
Ray, P.S. et al. A stress-responsive RNA switch regulates VEGFA expression. Nature 457, 915–919 (2009).
Chang, C.H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).This study establishes links between metabolism and post-transcriptional regulation that direct effector T cell function.
Venigalla, R.K. & Turner, M. RNA-binding proteins as a point of convergence of the PI3K and p38 MAPK pathways. Front. Immunol. 3, 398 (2012).
Mori, M. et al. Hippo signaling regulates microprocessor and links cell-density-dependent miRNA biogenesis to cancer. Cell 156, 893–906 (2014).
Trabucchi, M. et al. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature 459, 1010–1014 (2009).
Bronevetsky, Y. et al. T cell activation induces proteasomal degradation of Argonaute and rapid remodeling of the microRNA repertoire. J. Exp. Med. 210, 417–432 (2013).
Uehata, T. et al. Malt1-induced cleavage of regnase-1 in CD4+ helper T cells regulates immune activation. Cell 153, 1036–1049 (2013).
Iwasaki, H. et al. The IκB kinase complex regulates the stability of cytokine-encoding mRNA induced by TLR-IL-1R by controlling degradation of regnase-1. Nat. Immunol. 12, 1167–1175 (2011).
Cano, F., Miranda-Saavedra, D. & Lehner, P.J. RNA-binding E3 ubiquitin ligases: novel players in nucleic acid regulation. Biochem. Soc. Trans. 38, 1621–1626 (2010).
Cano, F. et al. The RNA-binding E3 ubiquitin ligase MEX-3C links ubiquitination with MHC-I mRNA degradation. EMBO J. 31, 3596–3606 (2012).
Maruyama, T. et al. Roquin-2 promotes ubiquitin-mediated degradation of ASK1 to regulate stress responses. Sci. Signal. 7, ra8 (2014).
Leung, A.K. et al. Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol. Cell 42, 489–499 (2011).
Shen, Z.J., Esnault, S. & Malter, J.S. The peptidyl-prolyl isomerase Pin1 regulates the stability of granulocyte-macrophage colony-stimulating factor mRNA in activated eosinophils. Nat. Immunol. 6, 1280–1287 (2005).
Sumazin, P. et al. An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell 147, 370–381 (2011).
Keene, J.D. RNA regulons: coordination of post-transcriptional events. Nat. Rev. Genet. 8, 533–543 (2007).
Masuda, K. et al. Arid5a controls IL-6 mRNA stability, which contributes to elevation of IL-6 level in vivo. Proc. Natl. Acad. Sci. USA 110, 9409–9414 (2013).
Ho, J.J. & Marsden, P.A. Competition and collaboration between RNA-binding proteins and microRNAs. Wiley Interdiscip. Rev. RNA 5, 69–86 (2014).
Brooks, S.A. & Blackshear, P.J. Tristetraprolin (TTP): interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim. Biophys. Acta 1829, 666–679 (2013).
Dai, W., Zhang, G. & Makeyev, E.V. RNA-binding protein HuR autoregulates its expression by promoting alternative polyadenylation site usage. Nucleic Acids Res. 40, 787–800 (2012).
Dassi, E. et al. Hyperconserved elements in vertebrate mRNA 3′-UTRs reveal a translational network of RNA-binding proteins controlled by HuR. Nucleic Acids Res. 41, 3201–3216 (2013).
Eiring, A.M. et al. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell 140, 652–665 (2010).Here a miRNA is found to act by a new mechanism; it functions as a decoy that blocks the interaction of an RBP with target transcripts.
Clark, M.B. et al. Genome-wide analysis of long noncoding RNA stability. Genome Res. 22, 885–898 (2012).
Niazi, F. & Valadkhan, S. Computational analysis of functional long noncoding RNAs reveals lack of peptide-coding capacity and parallels with 3′ UTRs. RNA 18, 825–843 (2012).
Hansen, T.B. et al. Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388 (2013).
Memczak, S. et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338 (2013).
Tay, Y., Rinn, J. & Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 505, 344–352 (2014).
Kretz, M. et al. Control of somatic tissue differentiation by the long noncoding RNA TINCR. Nature 493, 231–235 (2012).
Yoon, J.H. et al. LincRNA-p21 suppresses target mRNA translation. Mol. Cell 47, 648–655 (2012).
Makeyev, E.V., Zhang, J., Carrasco, M.A. & Maniatis, T. The microRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol. Cell 27, 435–448 (2007).
Yuan, J., Nguyen, C.K., Liu, X., Kanellopoulou, C. & Muljo, S.A. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science 335, 1195–1200 (2012).This article shows the ability of an RBP to reprogram hematopoietic cells, demonstrating the fundamental power of this layer of control.
Rounbehler, R.J. et al. Tristetraprolin impairs Myc-induced lymphoma and abolishes the malignant state. Cell 150, 563–574 (2012).
Heissmeyer, V. & Vogel, K.U. Molecular control of TFH-cell differentiation by Roquin family proteins. Immunol. Rev. 253, 273–289 (2013).
Akira, S. Regnase-1, a ribonuclease involved in the regulation of immune responses. Cold Spring Harb. Symp. Quant. Biol. 10.1101/sqb.2013.78.019877 (25 October 2013).
Yiakouvaki, A. et al. Myeloid cell expression of the RNA-binding protein HuR protects mice from pathologic inflammation and colorectal carcinogenesis. J. Clin. Invest. 122, 48–61 (2012).
Simarro, M. et al. The translational repressor T-cell intracellular antigen-1 (TIA-1) is a key modulator of Th2 and Th17 responses driving pulmonary inflammation induced by exposure to house dust mite. Immunol. Lett. 146, 8–14 (2012).
Baumjohann, D. & Ansel, K.M. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat. Rev. Immunol. 13, 666–678 (2013).
Li, Q.J. et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell 129, 147–161 (2007).
Ebert, P.J., Jiang, S., Xie, J., Li, Q.J. & Davis, M.M. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat. Immunol. 10, 1162–1169 (2009).
Baumjohann, D. et al. The microRNA cluster miR-17 approximately 92 promotes TFH cell differentiation and represses subset-inappropriate gene expression. Nat. Immunol. 14, 840–848 (2013).
Kang, S.G. et al. MicroRNAs of the miR-17 approximately 92 family are critical regulators of TFH differentiation. Nat. Immunol. 14, 849–857 (2013).
Jin, H.Y. et al. MicroRNA-17∼92 plays a causative role in lymphomagenesis by coordinating multiple oncogenic pathways. EMBO J. 32, 2377–2391 (2013).
Gracias, D.T. et al. The microRNA miR-155 controls CD8+ T cell responses by regulating interferon signaling. Nat. Immunol. 14, 593–602 (2013).
Willingham, A.T. et al. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science 309, 1570–1573 (2005).Refs. 92 and 93 highlight a structural role for noncoding RNA in signal-transduction complexes. It will be interesting to see whether further examples are found.
Sharma, S. et al. Dephosphorylation of the nuclear factor of activated T cells (NFAT) transcription factor is regulated by an RNA-protein scaffold complex. Proc. Natl. Acad. Sci. USA 108, 11381–11386 (2011).
Madhani, H.D. The frustrated gene: origins of eukaryotic gene expression. Cell 155, 744–749 (2013).
Reineke, L.C. & Lloyd, R.E. Diversion of stress granules and P-bodies during viral infection. Virology 436, 255–267 (2013).
Liu, S. et al. MCPIP1 restricts HIV infection and is rapidly degraded in activated CD4+ T cells. Proc. Natl. Acad. Sci. USA 110, 19083–19088 (2013).
Lin, R.J. et al. MCPIP1 ribonuclease exhibits broad-spectrum antiviral effects through viral RNA binding and degradation. Nucleic Acids Res. 41, 3314–3326 (2013).
Gao, G., Guo, X. & Goff, S.P. Inhibition of retroviral RNA production by ZAP, a CCCH-type zinc finger protein. Science 297, 1703–1706 (2002).
Hayakawa, S. et al. ZAPS is a potent stimulator of signaling mediated by the RNA helicase RIG-I during antiviral responses. Nat. Immunol. 12, 37–44 (2011).
Lee, H. et al. Zinc-finger antiviral protein mediates retinoic acid inducible gene I-like receptor-independent antiviral response to murine leukemia virus. Proc. Natl. Acad. Sci. USA 110, 12379–12384 (2013).
Li, Y., Lu, J., Han, Y., Fan, X. & Ding, S.W. RNA interference functions as an antiviral immunity mechanism in mammals. Science 342, 231–234 (2013).Refs. 101, 102 and 107 advance the understanding of the limited role of small interfering RNAs in antiviral responses.
Maillard, P.V. et al. Antiviral RNA interference in mammalian cells. Science 342, 235–238 (2013).
Cullen, B.R., Cherry, S. & Tenoever, B.R. Is RNA interference a physiologically relevant innate antiviral immune response in mammals? Cell Host Microbe 14, 374–378 (2013).
Cullen, B.R. MicroRNAs as mediators of viral evasion of the immune system. Nat. Immunol. 14, 205–210 (2013).
Trobaugh, D.W. et al. RNA viruses can hijack vertebrate microRNAs to suppress innate immunity. Nature 506, 245–248 (2014).
Jopling, C.L., Yi, M., Lancaster, A.M., Lemon, S.M. & Sarnow, P. Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science 309, 1577–1581 (2005).
Seo, G.J. et al. Reciprocal inhibition between intracellular antiviral signaling and the RNAi machinery in mammalian cells. Cell Host Microbe 14, 435–445 (2013).
Rodríguez Pulido, M., Serrano, P., Saiz, M. & Martinez-Salas, E. Foot-and-mouth disease virus infection induces proteolytic cleavage of PTB, eIF3a,b, and PABP RNA-binding proteins. Virology 364, 466–474 (2007).
Moon, S.L. et al. A noncoding RNA produced by arthropod-borne flaviviruses inhibits the cellular exoribonuclease XRN1 and alters host mRNA stability. RNA 18, 2029–2040 (2012).
Barnhart, M.D., Moon, S.L., Emch, A.W., Wilusz, C.J. & Wilusz, J. Changes in cellular mRNA stability, splicing, and polyadenylation through HuR protein sequestration by a cytoplasmic RNA virus. Cell Rep. 5, 909–917 (2013).
Lee, N., Pimienta, G. & Steitz, J.A. AUF1/hnRNP D is a novel protein partner of the EBER1 noncoding RNA of Epstein-Barr virus. RNA 18, 2073–2082 (2012).
Cazalla, D., Yario, T. & Steitz, J.A. Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science 328, 1563–1566 (2010).
Marcinowski, L. et al. Degradation of cellular mir-27 by a novel, highly abundant viral transcript is important for efficient virus replication in vivo. PLoS Pathog. 8, e1002510 (2012).
Lee, S. et al. Selective degradation of host microRNAs by an intergenic HCMV noncoding RNA accelerates virus production. Cell Host Microbe 13, 678–690 (2013).This article describes a viral noncoding RNA that is needed to promote the production of virus under lytic conditions and mediates the degradation of a host-cell miRNA.
McFarland, A.P. et al. The favorable IFNL3 genotype escapes mRNA decay mediated by AU-rich elements and hepatitis C virus–induced microRNAs. Nat. Immunol. 15, 72–79 (2014).
LaMonte, G. et al. Translocation of sickle cell erythrocyte microRNAs into Plasmodium falciparum inhibits parasite translation and contributes to malaria resistance. Cell Host Microbe 12, 187–199 (2012).
Acknowledgements
We thank M. Linterman, P. Katsikis, R. Newman and L. Webb for advice and comments on the manuscript. Supported by the Biotechnology and Biological Sciences Research Council, the Medical Research Council (M.T. and E.V.) and Leukaemia and Lymphoma Research (A.G.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Turner, M., Galloway, A. & Vigorito, E. Noncoding RNA and its associated proteins as regulatory elements of the immune system. Nat Immunol 15, 484–491 (2014). https://doi.org/10.1038/ni.2887
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2887
This article is cited by
-
Construction of Immune Infiltration-Related LncRNA Signatures Based on Machine Learning for the Prognosis in Colon Cancer
Biochemical Genetics (2023)
-
Expression and diagnostic values of MIAT, H19, and NRON long non-coding RNAs in multiple sclerosis patients
Egyptian Journal of Medical Human Genetics (2022)
-
Evaluating the utility of an immune checkpoint-related lncRNA signature for identifying the prognosis and immunotherapy response of lung adenocarcinoma
Scientific Reports (2022)
-
Immune checkpoints related-LncRNAs can identify different subtypes of lung cancer and predict immunotherapy and prognosis
Journal of Cancer Research and Clinical Oncology (2022)
-
Dendritic cell migration in inflammation and immunity
Cellular & Molecular Immunology (2021)