Abstract
Among the features that distinguish type 1 innate lymphoid cells (ILC1s) from natural killer (NK) cells is a gene signature indicative of 'imprinting' by cytokines of the TGF-β family. We studied mice in which ILC1s and NK cells lacked SMAD4, a signal transducer that facilitates the canonical signaling pathway common to all cytokines of the TGF-β family. While SMAD4 deficiency did not affect ILC1 differentiation, NK cells unexpectedly acquired an ILC1-like gene signature and were unable to control tumor metastasis or viral infection. Mechanistically, SMAD4 restrained non-canonical TGF-β signaling mediated by the cytokine receptor TGFβR1 in NK cells. NK cells from a SMAD4-deficient person affected by polyposis were also hyper-responsive to TGF-β. These results identify SMAD4 as a previously unknown regulator that restricts non-canonical TGF-β signaling in NK cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Marcus, A. et al. Recognition of tumors by the innate immune system and natural killer cells. Adv. Immunol. 122, 91–128 (2014).
Yu, X. et al. The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. eLife 3, 1–20 (2014).
Klose, C.S.N. et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 157, 340–356 (2014).
Constantinides, M.G., McDonald, B.D., Verhoef, P.A. & Bendelac, A. A committed precursor to innate lymphoid cells. Nature 508, 397–401 (2014).
Daussy, C. et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J. Exp. Med. 211, 563–577 (2014).
Constantinides, M.G. et al. PLZF expression maps the early stages of ILC1 lineage development. Proc. Natl. Acad. Sci. USA 112, 5123–5128 (2015).
Geiger, T.L. & Sun, J.C. Development and maturation of natural killer cells. Curr. Opin. Immunol. 39, 82–89 (2016).
Bernink, J.H. et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 14, 221–229 (2013).
Simoni, Y. et al. Human Innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity 46, 148–161 (2017).
Lim, A.I. et al. Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell 168, 1086–1100 e1010 (2017).
Sojka, D.K. et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife 3, e01659 (2014).
Gasteiger, G., Fan, X., Dikiy, S., Lee, S.Y. & Rudensky, A.Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350, 981–985 (2015).
Cortez, V.S. et al. Transforming growth factor-β signaling guides the differentiation of innate lymphoid cells in salivary glands. Immunity 44, 1127–1139 (2016).
Fuchs, A. et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity 38, 769–781 (2013).
Cortez, V.S., Fuchs, A., Cella, M., Gilfillan, S. & Colonna, M. Cutting edge: salivary gland NK cells develop independently of Nfil3 in steady-state. J. Immunol. 192, 4487–4491 (2014).
Mackay, L.K. et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352, 459–463 (2016).
Narni-Mancinelli, E. et al. Fate mapping analysis of lymphoid cells expressing the NKp46 cell surface receptor. Proc. Natl. Acad. Sci. USA 108, 18324–18329 (2011).
Robinette, M.L. et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat. Immunol. 16, 306–317 (2015).
Massagué, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 13, 616–630 (2012).
He, W. et al. Hematopoiesis controlled by distinct TIF1γ and Smad4 branches of the TGFβ pathway. Cell 125, 929–941 (2006).
Zhang, Y.E. Non-Smad signaling pathways of the TGF-β family. CSH Perspec. Biol. 9, 1–18 (2017).
Laouar, Y., Sutterwala, F.S., Gorelik, L. & Flavell, R.A. Transforming growth factor-β controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nat. Immunol. 6, 600–607 (2005).
Marcoe, J.P. et al. TGF-β is responsible for NK cell immaturity during ontogeny and increased susceptibility to infection during mouse infancy. Nat. Immunol. 13, 843–850 (2012).
Viel, S. et al. TGF-β inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal. 9, ra19 (2016).
Yang, X., Li, C., Herrera, P.L. & Deng, C.X. Generation of Smad4/Dpc4 conditional knockout mice. Genesis 32, 80–81 (2002).
Takeda, K. et al. IFN-γ production by lung NK cells is critical for the natural resistance to pulmonary metastasis of B16 melanoma in mice. J. Leukoc. Biol. 90, 777–785 (2011).
van Helden, M.J. et al. Terminal NK cell maturation is controlled by concerted actions of T-bet and Zeb2 and is essential for melanoma rejection. J. Exp. Med. 212, 2015–2025 (2015).
Lakshmikanth, T. et al. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J. Clin. Invest. 119, 1251–1263 (2009).
Gilfillan, S. et al. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J. Exp. Med. 205, 2965–2973 (2008).
Johnston, R.J. et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8+ T cell effector function. Cancer Cell 26, 923–937 (2014).
Chauvin, J.M. et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J. Clin. Invest. 125, 2046–2058 (2015).
Stanietsky, N. et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 106, 17858–17863 (2009).
Boles, K.S. et al. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur. J. Immunol. 39, 695–703 (2009).
Vidal, S.M. & Lanier, L.L. NK cell recognition of mouse cytomegalovirus-infected cells. Curr. Top. Microbiol. Immunol. 298, 183–206 (2006).
Miyaki, M. & Kuroki, T. Role of Smad4 (DPC4) inactivation in human cancer. Biochem. Biophys. Res. Commun. 306, 799–804 (2003).
Larsen Haidle, J. & Howe, J.R. Juvenile polyposis syndrome. GeneReviews 1–28 (1993).
Howe, J.R. et al. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science 280, 1086–1088 (1998).
Kim, B.G. et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature 441, 1015–1019 (2006).
Li, M.O. & Flavell, R.A. TGF-β: a master of all T cell trades. Cell 134, 392–404 (2008).
Gu, A.D. et al. A critical role for transcription factor Smad4 in T cell function that is independent of transforming growth factor β receptor signaling. Immunity 42, 68–79 (2015).
Huang, F. & Chen, Y.G. Regulation of TGF-β receptor activity. Cell Biosci. 2, 9 (2012).
Dadi, S. et al. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell 164, 365–377 (2016).
Gao, Y. et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. http://dx.doi.org/10.1038/ni.3800 (2017).
Schlenner, S.M. et al. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity 32, 426–436 (2010).
Cella, M. & Colonna, M. Cloning human natural killer cells. Methods Mol. Biol. 121, 1–4 (2000).
Acknowledgements
We thank the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine for help with genomic analysis; H.-R. Rodewald (DKZF, Heidelberg, Germany) for Il7rCre mice; and M. Rabinovitch (Stanford University, USA) for Bmpr2f/f mice. Supported by the US National Institutes of Health (U01 AI095542, R01 DE025884 and R01 DK103039 to M. Colonna; R01 CA176695 to M. Cella; and 5T32CA009547-30 to V.S.C.), the Crohn's & Colitis Foundation of America (274415) and Cancer Research Institute (J.K.B.).
Author information
Authors and Affiliations
Contributions
V.S.C., T.K.U., L.C-B., J.K.B., Q.W., S.G. and M. Cella performed experiments and analyzed the data; V.S.C., M.L.R., and M. Cella performed in silico analysis; A.J.W. provided materials and clinical expertise; V.S.C., M. Cella and M. Colonna designed the experiments; and V.S.C., S.G., M. Cella and M. Colonna wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
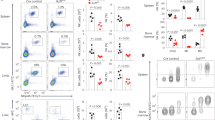
Supplementary Figure 1 Marker expression by NK cells and from Smad4f/f and Smad4f/fNcr1iCre mice.
(a) Representative plots displaying pre-sort and post-sort purity of splenic NK cells from Smad4f/f and Smad4f/f x Ncr1iCre mice. (b) Quantification of surface expression of the indicated markers by splenic NK cells from Smad4f/f and Smad4f/f x Ncr1iCre mice. (c) Representative histograms showing expression of the indicated proteins by splenic NK cells from WT and SMAD4-deficient mice. (d) Representative plots and quantification of siLP ILC3 populations in WT and SMAD4-deficient mice. (e) Representative plots and quantification of intracellular IL-22 by siLP NKp46+ ILC3 after stimulation by IL-23. (f) Representative histograms of cell surface expression of CD49a in NKp46− or NKp46+ NK cells from the bone marrow of Smad4+/+ x Il7rCre and Smad4f/f x Il7rCre mice. **P<0.01, ***P < 0.001; (unpaired Student's t-test). Data are pooled from four (b) or two (d,e,f) independent experiments with 1-2 mice of each genotype for each independent experiment (error bars represent mean ± s.d. in b,d,e).
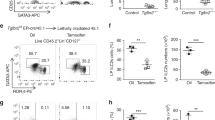
Supplementary Figure 2 Apoptosis of Smad4f/f and Smad4f/fNcr1iCre NK cells during MCMV infection or in vitro culture.
(a) Representative plots of Ly49H+ and Ly49H− splenic NK cells (gated on CD3−CD19−) from naïve Smad4f/f and Smad4f/f x Ncr1iCre mice. (b) Ly49H+ splenic NK cells from Smad4f/f and Smad4f/f x Ncr1iCre mice positive for Annexin V at days 3 and 6 of MCMV infection. (c) Splenic NK cells from Smad4f/f and Smad4f/f x Ncr1iCre mice positive for Annexin V ex vivo or after 24 and 48 h of culture with IL-2. Data are pooled (b, c) from two independent experiments with at least 1-2 mice of each genotype for each independent experiment (error bars represent mean ± s.d. in b, c).
Supplementary Figure 3 Culture of Smad4f/f and Smad4f/fNcr1iCre NK cells with cytokines of the TGF-β-family.
Representative histograms and quantification of CD49a, CD73, and TRAIL surface expression by (a) Smad4f/f and (b) Smad4f/f x Ncr1iCre NK cells after 48 h of culture with TGF-β1 + IL-2, BMP4 + IL-2, activin A + IL-2, or IL-2 alone. **P<0.01, ***P < 0.001 (unpaired Student's t-test). Data are pooled from three independent experiments with 1-2 mice of each genotype for each independent experiment (error bars represent mean ± s.d.).
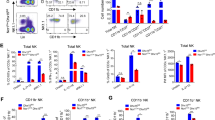
Supplementary Figure 4 Effect of deficiency in SMAD4, DKO deficiency, deficiency in TGFβR2 or deficiency in BMPR2 on conventional NK cells and SG ILC1s.
(a) Representative histograms of TGFβR2 expression by splenic NK cells from Smad4f/f, Smad4f/f x Ncr1iCre, DKO, and Tgfbr2f/f x Ncr1iCre mice ex vivo. (b) Representative histograms intracellular phospho-SMAD2/3 and phospho-SMAD1/8 in splenic NK cells after 60 min of culture with TGF-β1 and IL-2. (c) Representative histograms and quantification of CD49a expression by SG ILC1 from Smad4f/f, Smad4f/f x Ncr1iCre, DKO, and Tgfbr2f/f x Ncr1iCre mice. (d) Arbitrary units of gene expression of type II receptors by splenic NK cells of wild-type mice (Immgen.org). (e) Representative histograms of cell surface expression of CD49a, CD73, and TRAIL (after 48 h of culture with IL-2) by splenic NK cells from Smad4f/f, Smad4f/f x Ncr1iCre, Tgfbr2f/f x Ncr1iCre, and Bmpr2f/f x Ncr1iCre mice. (f) Quantification of Annexin V+ NK cells from Smad4f/f, Smad4f/f x Ncr1iCre, DKO, and Tgfbr2f/f x Ncr1iCre mice after 48 h of culture with IL-2 or TGF-β1 plus IL-2 and DMSO or SB431542. ***P < 0.001 (unpaired Student's t-test). Data are pooled from at least two independent experiments with 1-2 mice of each genotype for each independent experiment (error bars represent mean ± s.d.).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 906 kb)
Rights and permissions
About this article
Cite this article
Cortez, V., Ulland, T., Cervantes-Barragan, L. et al. SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-β signaling. Nat Immunol 18, 995–1003 (2017). https://doi.org/10.1038/ni.3809
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3809
This article is cited by
-
Natural killer cell therapies
Nature (2024)
-
Rapid functional impairment of natural killer cells following tumor entry limits anti-tumor immunity
Nature Communications (2024)
-
Perinatal murine cytomegalovirus infection reshapes the transcriptional profile and functionality of NK cells
Nature Communications (2023)
-
Innate lymphoid cells and innate-like T cells in cancer — at the crossroads of innate and adaptive immunity
Nature Reviews Cancer (2023)
-
TGFβ control of immune responses in cancer: a holistic immuno-oncology perspective
Nature Reviews Immunology (2023)