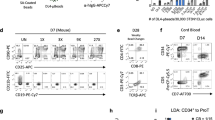
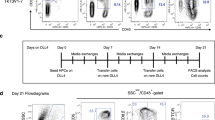
Abstract
Embryonic stem cells (ESCs) have the potential to serve as a renewable source of transplantable tissue-specific stem cells. However, the molecular cues necessary to direct the differentiation of ESCs toward specific cell lineages remain obscure. Here we report the successful induction of ESC differentiation into mature functional T lymphocytes with a simple in vitro coculture system. The directed differentiation of ESCs into T cells required the engagement of Notch receptors by Delta-like 1 ligand (DL1) expressed on the OP9-DL1 stromal cell line. We found a normal program of T cell differentiation in ESC–OP9-DL1 cell cocultures. ESC-derived T cell progenitors effectively reconstituted the T cell compartment of immunodeficient mice, enabling an effective response to a viral infection. These findings provide a powerful tool for the molecular analysis of T cell development and open new avenues for the development of immunotherapeutic approaches using defined sources of stem cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Doetschman, T.C., Eistetter, H., Katz, M., Schmidt, W. & Kemler, R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J. Embryol. Exp. Morphol. 87, 27–45 (1985).
Kennedy, M. et al. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature 386, 488–493 (1997).
Nakano, T., Kodama, H. & Honjo, T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science 265, 1098–1101 (1994).
Nakano, T. Lymphohematopoietic development from embryonic stem cells in vitro . Semin. Immunol. 7, 197–203 (1995).
Cho, S.K. et al. Functional characterization of B lymphocytes generated in vitro from embryonic stem cells. Proc. Natl. Acad. Sci. USA 96, 9797–9802 (1999).
Keller, G.M. In vitro differentiation of embryonic stem cells. Curr. Opin. Cell Biol. 7, 862–869 (1995).
Kaufman, M.H., Robertson, E.J., Handyside, A.H. & Evans, M.J. Establishment of pluripotential cell lines from haploid mouse embryos. J. Embryol. Exp. Morphol. 73, 249–261 (1983).
Orkin, S.H. & Morrison, S.J. Stem-cell competition. Nature 418, 25–27 (2002).
de Pooter, R.F., Cho, S.K., Carlyle, J.R. & Zúñiga-Pflücker, J.C. In vitro generation of T lymphocytes from embryonic stem cell-derived prehematopoietic progenitors. Blood 102, 1649–1653 (2003).
Schmitt, T.M. & Zúñiga-Pflücker, J.C. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro . Immunity 17, 749–756 (2002).
Pui, J.C. et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity 11, 299–308 (1999).
Radtke, F. et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10, 547–558 (1999).
Wolfer, A., Wilson, A., Nemir, M., MacDonald, H.R. & Radtke, F. Inactivation of Notch1 impairs VDJb rearrangement and allows pre-TCR-independent survival of early ab lineage thymocytes. Immunity 16, 869–879 (2002).
Washburn, T. et al. Notch activity influences the ab versus gd T cell lineage decision. Cell 88, 833–843 (1997).
Robey, E. et al. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell 87, 483–492 (1996).
Izon, D.J. et al. Notch1 regulates maturation of CD4+ and CD8+ thymocytes by modulating TCR signal strength. Immunity 14, 253–264 (2001).
Fowlkes, B.J. & Robey, E.A. A reassessment of the effect of activated Notch1 on CD4 and CD8 T cell development. J. Immunol. 169, 1817–1821 (2002).
Deftos, M.L., Huang, E., Ojala, E.W., Forbush, K.A. & Bevan, M.J. Notch1 signaling promotes the maturation of CD4 and CD8 SP thymocytes. Immunity 13, 73–84 (2000).
Engel, I., Johns, C., Bain, G., Rivera, R.R. & Murre, C. Early thymocyte development is regulated by modulation of E2A protein activity. J. Exp. Med. 194, 733–745 (2001).
Barndt, R.J., Dai, M. & Zhuang, Y. Functions of E2A-HEB heterodimers in T-cell development revealed by a dominant negative mutation of HEB. Mol. Cell. Biol. 20, 6677–6685 (2000).
Scott, E.W., Simon, M.C., Anastasi, J. & Singh, H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science 265, 1573–1577 (1994).
Ting, C.N., Olson, M.C., Barton, K.P. & Leiden, J.M. Transcription factor GATA3 is required for development of the T-cell lineage. Nature 384, 474–478 (1996).
Williams, R.L. et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 336, 684–687 (1988).
Shortman, K. & Wu, L. Early T lymphocyte progenitors. Annu. Rev. Immunol. 14, 29–47 (1996).
Rothenberg, E.V., Telfer, J.C. & Anderson, M.K. Transcriptional regulation of lymphocyte lineage commitment. Bioessays 21, 726–742 (1999).
Anderson, M.K., Weiss, A.H., Hernandez-Hoyos, G., Dionne, C.J. & Rothenberg, E.V. Constitutive expression of PU.1 in fetal hematopoietic progenitors blocks T cell development at the pro-T cell stage. Immunity 16, 285–296 (2002).
Zhuang, Y., Soriano, P. & Weintraub, H. The helix-loop-helix gene E2A is required for B cell formation. Cell 79, 875–884 (1994).
Bain, G. et al. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell 79, 885–892 (1994).
Peschon, J.J. et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 180, 1955–1960 (1994).
Mombaerts, P. et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–877 (1992).
Tomita, K. et al. The bHLH gene Hes1 is essential for expansion of early T cell precursors. Genes Dev. 13, 1203–1210 (1999).
Anderson, M.K. et al. Definition of regulatory network elements for T cell development by perturbation analysis with PU.1 and GATA3. Dev. Biol. 246, 103–121 (2002).
Kyba, M., Perlingeiro, R.C. & Daley, G.Q. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell 109, 29–37 (2002).
Robertson, E.J. Derivation and maintenance of embryonic stem cell cultures. Methods Mol. Biol. 75, 173–184 (1997).
Cho, S.K. & Zúñiga-Pflücker, J.C. Development of lymphoid lineages from embryonic stem cells in vitro . Methods Enzymol. 365, 158–169 (2003).
Rodewald, H.-R., Kretzschmar, K., Takeda, S., Hohl, C. & Dessing, M. Identification of pro-thymocytes in murine fetal blood: T lineage commitment can precede thymus colonization. EMBO J. 13, 4229–4240 (1994).
Carlyle, J.R. & Zúñiga-Pflücker, J.C. Requirement for the thymus in ab T lymphocyte lineage commitment. Immunity 9, 187–197 (1998).
Godfrey, D.I., Kennedy, J., Suda, T. & Zlotnik, A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple negative adult mouse thymocytes defined by CD44 and CD25 expression. J. Immunol. 150, 4244–4252 (1993).
Acknowledgements
We thank P. Poussier for critical review of the manuscript and G. Knowles for assistance in cell sorting. Supported by Canadian Institutes of Health Research (R.F.d.P. and J.C.Z.-P.), the National Cancer Institute of Canada and the Canadian Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Schmitt, T., de Pooter, R., Gronski, M. et al. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol 5, 410–417 (2004). https://doi.org/10.1038/ni1055
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1055
This article is cited by
-
Generation and clinical potential of functional T lymphocytes from gene-edited pluripotent stem cells
Experimental Hematology & Oncology (2022)
-
Regeneration of immunocompetent B lymphopoiesis from pluripotent stem cells guided by transcription factors
Cellular & Molecular Immunology (2021)
-
Pluripotent stem cell-derived CD19-CAR iT cells effectively eradicate B-cell lymphoma in vivo
Cellular & Molecular Immunology (2021)
-
Guiding T lymphopoiesis from pluripotent stem cells by defined transcription factors
Cell Research (2020)
-
Multiscale engineering of immune cells and lymphoid organs
Nature Reviews Materials (2019)